Desymmetrization of Cyclopropenes via the Potassium-Templated Diastereoselective 7- exo- trig Cycloaddition of Tethered Amino Alcohols toward Enantiopure Cyclopropane-Fused Oxazepanones with Antimycobacterial Activity
- PMID: 29696970
- PMCID: PMC6693504
- DOI: 10.1021/acs.joc.8b00640
Desymmetrization of Cyclopropenes via the Potassium-Templated Diastereoselective 7- exo- trig Cycloaddition of Tethered Amino Alcohols toward Enantiopure Cyclopropane-Fused Oxazepanones with Antimycobacterial Activity
Abstract
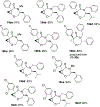
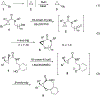
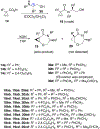
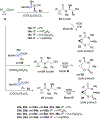
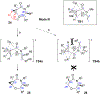
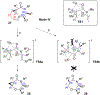
A strain-release-driven, cation-templated intramolecular nucleophilic addition of tethered alkoxides to prochiral cyclopropenes is described. Employment of chiral β- and γ-amino alkoxides allowed for highly diastereoselective assembly of a small series of enantiopure cyclopropane-fused oxazepanones. It was shown that the chiral center at C-4 plays a crucial role in controlling desymmetrization of the cyclopropenyl moiety, instigated by a profound potassium-templated effect. The preliminary biological activities of the new cyclopropane-fused medium heterocycles against Gram-positive bacteria, Gram-negative bacteria, mycobacteria, cancer cells, and fungus were evaluated.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


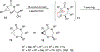





References
-
-
For recent reviews, see:
- Edwards A; Rubina M; Rubin M Nucleophilic Addition of Cyclopropenes. Curr. Org. Chem 2016, 20, 1862–1877.
- Vicente R Recent Progresses towards the Strengthening of Cyclopropene Chemistry. Synthesis 2016, 48, 2343–2360.
- Zhu Z-B; Wei Y; Shi M Recent Developments of Cyclopropene Chemistry. Chem. Soc. Rev 2011, 40, 5534–5563. - PubMed
-
-
-
See, for example:
- Zhang M; Guo J; Gong Y Highly Regioselective Cascade Formal Nucleophilic Substitution and Aldol Condensation of 2-Aroyl-1-chlorocyclopropanecarboxylic Esters. Eur. J. Org. Chem 2014, 2014, 1942–1950.
- Zhang M; Luo F; Gong Y Stereoselective Cascade Formal Nucleophilic Substitution and Mannich Reaction of Ethyl 2-Aroyl-1-chlorocyclopropanecarboxylates. J. Org. Chem 2014, 79, 1335–1343. - PubMed
- Hu J; Zhang M; Gong Y Cycloaddition Reactions of Alkyl Cyclopropenecarboxylates Generated in situ with Nitrones: Construction of Substituted Pyrroles and 1,2-Oxazinanes. Eur. J. Org. Chem 2015, 2015, 1970–1978.
-
-
-
See, for example:
- Shavrin KN; Gvozdev VD; Budanov DV; Yurov SV; Nefedov OM 1-(Alk-1-ynyl)cyclopropenes: Synthesis by Interaction of 1-(Alk-1-ynyl)-1-halocyclopropanes with Lithium N,N-Dialkylamides and Subsequent Additions of the Latter. Mendeleev Commun 2006, 16, 73–76.
- Huang Z; Hu J; Gong Y Formation and Aromatization of Strained Bicyclic Pyrazolidines via Tandem Reaction of Alkyl 2-Aroyl-1-chlorocyclopropanecarboxylates with Acylhydrazones. Org. Biomol. Chem 2015, 13, 8561–8566. - PubMed
- Zhu Y; Gong Y Construction of 2-Pyrone Skeleton via Domino Sequence between 2-Acyl-1-Chlorocyclopropanecarboxylate and Amines. J. Org. Chem 2015, 80, 490–498. - PubMed
- Shavrin KN; Gvozdev VD; Nefedov OM 5-Methylenehexahydropyrrolo [1,2-a]imidazoles and 6-Methyleneoctahydropyrrolo[1,2-a]pyrimidines in the Reactions of 1-(Alk-1-ynyl)-1-chlorocyclopropanes with Lithium Derivatives of 1,2- and 1,3-Diaminoalkanes. Mendeleev Commun. 2008, 18, 300–301.
-
-
- Shavrin KN; Gvozdev VD; Nefedov OM Reactions of 1-(Alk-1-ynyl)-1-chlorocyclopropanes with Arenethiols and Alkanethiols in Dimethyl Sulfoxide in the Presense of KOH. Russ. Chem. Bull 2009, 58, 2432–2436.
-
- Zhang M; Gong Y; Wang W A Two-Step Sequence to Ethyl α-Fluorocyclopropanecarboxylates Through MIRC Reaction of Ethyl Dichloroacetate and Highly Regioselective Fluorination. Eur. J. Org. Chem 2013, 2013, 7372–7381.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

