4'-Ethynyl-2-fluoro-2'-deoxyadenosine, MK-8591: a novel HIV-1 reverse transcriptase translocation inhibitor
- PMID: 29697468
- PMCID: PMC6449048
- DOI: 10.1097/COH.0000000000000467
4'-Ethynyl-2-fluoro-2'-deoxyadenosine, MK-8591: a novel HIV-1 reverse transcriptase translocation inhibitor
Abstract
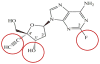
Purpose of review: 4'-Ethynyl-2-fluoro-2'-deoxyadenosine (EFdA) is a nucleoside reverse transcriptase inhibitor (NRTI) with a novel mechanism of action, unique structure, and amongst NRTIs, unparalleled anti-HIV-1 activity. We will summarize its structure and function, antiviral activity, resistance profile, and potential as an antiretroviral for use in the treatment and preexposure prophylaxis of HIV-1 infection.
Recent findings: EFdA is active against wild-type (EC50 as low as 50 pmol/l) and most highly NRTI-resistant viruses. The active metabolite, EFdA-triphosphate, has been shown to have a prolonged intracellular half-life in human and rhesus (Rh) blood cells. As a result, single drug doses tested in simian immunodeficiency virus mac251-infected Rh macaques and HIV-1-infected individuals exhibited robust antiviral activity of 7-10 days duration. Preclinical studies of EFdA as preexposure prophylaxis in the Rh macaque/simian/human immunodeficiency virus low-dose intrarectal challenge model have shown complete protection when given in clinically relevant doses.
Summary: EFdA is a novel antiretroviral with activity against both wild-type and NRTI-resistant viruses. As a result of the prolonged intracellular half-life of its active moiety, it is amenable to flexibility in dosing of at least daily to weekly and perhaps longer.
Conflict of interest statement
Conflicts of interest: M.M. is a paid consultant to Merck Research Laboratories. His research program receives grant support from Merck Laboratories. Merck, Sharp, and Dohme has also provided him with honoraria for serving as a plenary speaker at MSD organized symposia. He also receives grant support from Gilead Sciences and GlaxoSmithKline/ViiV. He is also a member of the Gilead Speakers Bureau.
S.G.S. has served as a paid consultant to Merck Research Laboratories.
Figures
References
-
- Ohrui H. 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine, a nucleoside reverse transcriptase inhibitor, is highly potent against all human immunodeficiency viruses type 1 and has low toxicity. Chem Rec. 2006;6(3):133–43. - PubMed
-
- Ohrui H, Kohgo S, Hayakawa H, Kodama E, Matsuoka M, Nakata T, et al. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine: a nucleoside reverse transcriptase inhibitor with highly potent activity against all HIV-1 strains, favorable toxic profiles and stability in plasma. Nucleic Acids Symp Ser (Oxf) 2006;(50):1–2. - PubMed
-
- Kawamoto A, Kodama E, Sarafianos SG, Sakagami Y, Kohgo S, Kitano K, et al. 2′-deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol. 2008;40(11):2410–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

