Mice lacking Kcns1 in peripheral neurons show increased basal and neuropathic pain sensitivity
- PMID: 29697531
- PMCID: PMC6053330
- DOI: 10.1097/j.pain.0000000000001255
Mice lacking Kcns1 in peripheral neurons show increased basal and neuropathic pain sensitivity
Abstract
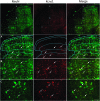
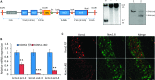
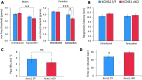
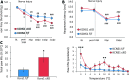
Voltage-gated potassium (Kv) channels are increasingly recognised as key regulators of nociceptive excitability. Kcns1 is one of the first potassium channels to be associated with neuronal hyperexcitability and mechanical sensitivity in the rat, as well as pain intensity and risk of developing chronic pain in humans. Here, we show that in mice, Kcns1 is predominantly expressed in the cell body and axons of myelinated sensory neurons positive for neurofilament-200, including Aδ-fiber nociceptors and low-threshold Aβ mechanoreceptors. In the spinal cord, Kcns1 was detected in laminae III to V of the dorsal horn where most sensory A fibers terminate, as well as large motoneurons of the ventral horn. To investigate Kcns1 function specifically in the periphery, we generated transgenic mice in which the gene is deleted in all sensory neurons but retained in the central nervous system. Kcns1 ablation resulted in a modest increase in basal mechanical pain, with no change in thermal pain processing. After neuropathic injury, Kcns1 KO mice exhibited exaggerated mechanical pain responses and hypersensitivity to both noxious and innocuous cold, consistent with increased A-fiber activity. Interestingly, Kcns1 deletion also improved locomotor performance in the rotarod test, indicative of augmented proprioceptive signalling. Our results suggest that restoring Kcns1 function in the periphery may be of some use in ameliorating mechanical and cold pain in chronic states.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

