A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 29697768
- PMCID: PMC5982716
- DOI: 10.1093/cid/ciy185
A Fungal Immunotherapeutic Vaccine (NDV-3A) for Treatment of Recurrent Vulvovaginal Candidiasis-A Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Background: Recurrent vulvovaginal candidiasis (RVVC) is a problematic form of mucosal Candida infection, characterized by repeated episodes per year. Candida albicans is the most common cause of RVVC. Currently, there are no immunotherapeutic treatments for RVVC.
Methods: This exploratory randomized, double-blind, placebo-controlled trial evaluated an immunotherapeutic vaccine (NDV-3A) containing a recombinant C. albicans adhesin/invasin protein for prevention of RVVC.
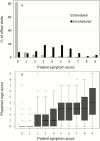
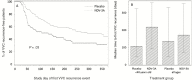
Results: The study in 188 women with RVVC (n = 178 evaluable) showed that 1 intramuscular dose of NDV-3A was safe and generated rapid and robust B- and T-cell immune responses. Post hoc exploratory analyses revealed a statistically significant increase in the percentage of symptom-free patients at 12 months after vaccination (42% vaccinated vs 22% placebo; P = .03) and a doubling in median time to first symptomatic episode (210 days vaccinated vs 105 days placebo) for the subset of patients aged <40 years (n = 137). The analysis of evaluable patients, which combined patients aged <40 years (77%) and ≥40 years (23%), trended toward a positive impact of NDV-3A versus placebo (P = .099).
Conclusions: In this unprecedented study of the effectiveness of a fungal vaccine in humans, NDV-3A administered to women with RVVC was safe and highly immunogenic and reduced the frequency of symptomatic episodes of vulvovaginal candidiasis for up to 12 months in women aged <40 years. These results support further development of NDV-3A vaccine and provide guidance for meaningful clinical endpoints for immunotherapeutic management of RVVC.
Clinical trials registration: NCT01926028.
Figures

Comment in
-
A Therapeutic Vaccine for Recurrent Vulvovaginal Candidiasis.Clin Infect Dis. 2018 Jun 1;66(12):1937-1939. doi: 10.1093/cid/ciy188. Clin Infect Dis. 2018. PMID: 29697770 Free PMC article. No abstract available.
References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med 2012; 4:165rv13. - PubMed
-
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2016; 214:15–21. - PubMed
-
- Marchaim D, Lemanek L, Bheemreddy S, Kaye KS, Sobel JD. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet Gynecol 2012; 120:1407–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

