Organization of the core respiratory network: Insights from optogenetic and modeling studies
- PMID: 29698394
- PMCID: PMC5940240
- DOI: 10.1371/journal.pcbi.1006148
Organization of the core respiratory network: Insights from optogenetic and modeling studies
Abstract
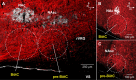
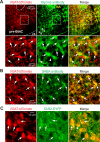
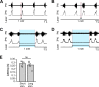
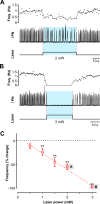
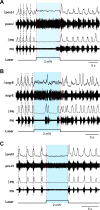
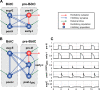
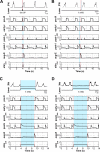
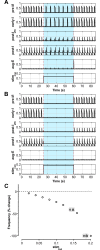
The circuit organization within the mammalian brainstem respiratory network, specifically within and between the pre-Bötzinger (pre-BötC) and Bötzinger (BötC) complexes, and the roles of these circuits in respiratory pattern generation are continuously debated. We address these issues with a combination of optogenetic experiments and modeling studies. We used transgenic mice expressing channelrhodopsin-2 under the VGAT-promoter to investigate perturbations of respiratory circuit activity by site-specific photostimulation of inhibitory neurons within the pre-BötC or BötC. The stimulation effects were dependent on the intensity and phase of the photostimulation. Specifically: (1) Low intensity (≤ 1.0 mW) pulses delivered to the pre-BötC during inspiration did not terminate activity, whereas stronger stimulations (≥ 2.0 mW) terminated inspiration. (2) When the pre-BötC stimulation ended in or was applied during expiration, rebound activation of inspiration occurred after a fixed latency. (3) Relatively weak sustained stimulation (20 Hz, 0.5-2.0 mW) of pre-BötC inhibitory neurons increased respiratory frequency, while a further increase of stimulus intensity (> 3.0 mW) reduced frequency and finally (≥ 5.0 mW) terminated respiratory oscillations. (4) Single pulses (0.2-5.0 s) applied to the BötC inhibited rhythmic activity for the duration of the stimulation. (5) Sustained stimulation (20 Hz, 0.5-3.0 mW) of the BötC reduced respiratory frequency and finally led to apnea. We have revised our computational model of pre-BötC and BötC microcircuits by incorporating an additional population of post-inspiratory inhibitory neurons in the pre-BötC that interacts with other neurons in the network. This model was able to reproduce the above experimental findings as well as previously published results of optogenetic activation of pre-BötC or BötC neurons obtained by other laboratories. The proposed organization of pre-BötC and BötC circuits leads to testable predictions about their specific roles in respiratory pattern generation and provides important insights into key circuit interactions operating within brainstem respiratory networks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Richter DW. Neural regulation of respiration: rhythmogenesis and afferent control Comprehensive Human Physiology. Springer, Berlin, Heidelberg; 1996. pp. 2079–2095. doi: 10.1007/978-3-642-60946-6_106 - DOI
-
- Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiol Bethesda. 2014;29: 58–71. doi: 10.1152/physiol.00035.2013 - DOI - PMC - PubMed
-
- Rybak IA, Shevtsova NA, Paton JFR, Dick TE, St.-John WM, Mörschel M, et al. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol. 2004;143: 307–319. doi: 10.1016/j.resp.2004.03.020 - DOI - PubMed
-
- Rybak IA, Abdala APL, Markin SN, Paton JFR, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res. 2007;165: 201–220. doi: 10.1016/S0079-6123(06)65013-9 - DOI - PMC - PubMed
-
- Rybak IA, O’Connor R, Ross A, Shevtsova NA, Nuding SC, Segers LS, et al. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100: 1770–1799. doi: 10.1152/jn.90416.2008 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

