Celecoxib use and circulating oxylipins in a colon polyp prevention trial
- PMID: 29698447
- PMCID: PMC5919576
- DOI: 10.1371/journal.pone.0196398
Celecoxib use and circulating oxylipins in a colon polyp prevention trial
Abstract
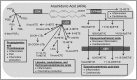
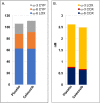
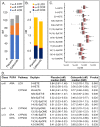
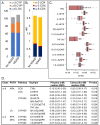
Drugs that inhibit cyclooxygenase (COX)-2 and the metabolism of arachidonic acid (ARA) to prostaglandin E2 are potent anti-inflammatory agents used widely in the treatment of joint and muscle pain. Despite their benefits, daily use of these drugs has been associated with hypertension, cardiovascular and gastrointestinal toxicities. It is now recognized that ARA is metabolized to a number of bioactive oxygenated lipids (oxylipins) by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) enzymes. Currently, the contribution of individual variability in ARA metabolism in response to the COX-2 inhibitors and potential adverse effects remains poorly understood. Using patient samples from the randomized, placebo-controlled phase III selenium/celecoxib (Sel/Cel) trial for the prevention of colorectal adenomatous polyps, we analyzed plasma concentrations of 74 oxylipins in a subset of participants who received celecoxib (n = 90) or placebo (n = 95). We assessed the effect of celecoxib (with and without low dose aspirin) on circulating oxylipins and systolic blood pressure (SBP). Individual CYP450- and LOX- but not COX-derived metabolites were higher with celecoxib than placebo (P<0.05) and differences were greater among non-aspirin users. LOX derived 5- and 8-HETE were elevated with celecoxib and positively associated with systolic blood pressure (P = 0.011 and P = 0.019 respectively). 20-HETE, a prohypertensive androgen-sensitive CYP450 metabolite was higher with celecoxib absent aspirin and was positively associated with SBP in men (P = 0.040) but not women. Independent of celecoxib or aspirin, LOX derived metabolites from ARA were strongly associated with SBP including 5- and 8-HETE. These findings support oxylipins, particularly the ARA LOX-derived, in blood pressure control and indicate that pharmacologic inhibition of COX-2 has effects on LOX and CYP450 ARA metabolism that contribute to hypertension in some patients.
Conflict of interest statement
Figures


Similar articles
-
Celecoxib for the prevention of colorectal adenomatous polyps.N Engl J Med. 2006 Aug 31;355(9):885-95. doi: 10.1056/NEJMoa061652. N Engl J Med. 2006. PMID: 16943401 Clinical Trial.
-
A Protective Role for Arachidonic Acid Metabolites against Advanced Colorectal Adenoma in a Phase III Trial of Selenium.Nutrients. 2021 Oct 29;13(11):3877. doi: 10.3390/nu13113877. Nutrients. 2021. PMID: 34836131 Free PMC article. Clinical Trial.
-
Targeted lipidomics uncovers oxylipin perturbations and potential circulation biomarkers in Bietti's crystalline dystrophy.Graefes Arch Clin Exp Ophthalmol. 2024 Dec;262(12):3773-3786. doi: 10.1007/s00417-024-06554-2. Epub 2024 Jul 4. Graefes Arch Clin Exp Ophthalmol. 2024. PMID: 38963460
-
Current perspective on the cardiovascular effects of coxibs.Cleve Clin J Med. 2002;69 Suppl 1:SI47-52. doi: 10.3949/ccjm.69.suppl_1.si47. Cleve Clin J Med. 2002. PMID: 12086293 Review.
-
COX-2 inhibition and colorectal cancer.Semin Oncol. 2004 Apr;31(2 Suppl 7):12-21. doi: 10.1053/j.seminoncol.2004.03.041. Semin Oncol. 2004. PMID: 15252926 Review.
Cited by
-
The Role of Cyclooxygenase-2 in Colorectal Cancer.Int J Med Sci. 2020 Apr 27;17(8):1095-1101. doi: 10.7150/ijms.44439. eCollection 2020. Int J Med Sci. 2020. PMID: 32410839 Free PMC article. Review.
-
Ibuprofen alters epoxide hydrolase activity and epoxy-oxylipin metabolites associated with different metabolic pathways in murine livers.Sci Rep. 2021 Mar 29;11(1):7042. doi: 10.1038/s41598-021-86284-1. Sci Rep. 2021. PMID: 33782432 Free PMC article.
-
Oxylipins as Biomarkers for Aromatase Inhibitor-Induced Arthralgia (AIA) in Breast Cancer Patients.Metabolites. 2023 Mar 20;13(3):452. doi: 10.3390/metabo13030452. Metabolites. 2023. PMID: 36984892 Free PMC article.
References
-
- Jiang J, Dingledine R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci. 2013;34(7):413–23. Epub 2013/06/26. doi: 10.1016/j.tips.2013.05.003 . - DOI - PMC - PubMed
-
- Moreira L, Castells A. Cyclooxygenase as a target for colorectal cancer chemoprevention. Curr Drug Targets. 2011;12(13):1888–94. . - PubMed
-
- Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146(5):376–89. . - PubMed
-
- Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, et al. Cardiovascular Risk of Celecoxib in 6 Randomized Placebo-Controlled Trials. The Cross Trial Safety Analysis. 2008;117(16):2104–13. doi: 10.1161/circulationaha.108.764530 - DOI - PMC - PubMed
-
- Bibbins-Domingo K, Force USPST. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(12):836–45. doi: 10.7326/M16-0577 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

