Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice
- PMID: 29698465
- PMCID: PMC5919537
- DOI: 10.1371/journal.pone.0196579
Barley β-glucan improves metabolic condition via short-chain fatty acids produced by gut microbial fermentation in high fat diet fed mice
Abstract
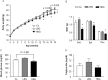
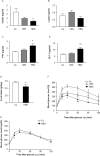
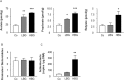
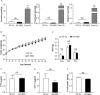
Dietary intake of barley β-glucan (BG) is known to affect energy metabolism. However, its underlying mechanism remains poorly understood because studies have presented inconsistent results, with both positive and negative effects reported in terms of satiety, energy intake, weight loss, and glycemic control. The objective of this study was to clarify the physiological role underlying the metabolic benefits of barley BG using a mouse model of high fat diet (HFD)-induced obesity. Male 4-wk-old C57BL/6J mice were fed an HFD with 20% barley flour containing either high BG (HBG; 2% BG) or low BG (LBG; 0.6% BG) levels under conventional and germ-free (GF) conditions for 12 wks. In addition, mice were fed either an HFD with 5% cellulose (HFC; high fiber cellulose) or 5% barley BG (HFB; high fiber β-glucan) for 12 wks. Then, metabolic parameters, gut microbial compositions, and the production of fecal short-chain fatty acids (SCFAs) were analyzed. The weight gain and fat mass of HBG-fed mice were lower than those of control mice at 16-wk-old. Moreover, the secretion of the gut hormones PYY and GLP-1 increased in HBG-fed mice, thereby reducing food intake and improving insulin sensitivity by changing the gut microbiota and increasing SCFAs (especially, butyrate) under conventional condition. These effects in HBG-fed mice were abolished under GF conditions. Moreover, the HFB diets also increased PYY and GLP-1 secretion, and decreased food intake compared with that in HFC-fed mice. These results suggest that the beneficial metabolic effects of barley BG are primary due to the suppression of appetite and improvement of insulin sensitivity, which are induced by gut hormone secretion promoted via gut microbiota-produced SCFAs.
Conflict of interest statement
Figures
References
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006; 444: 840–846. doi: 10.1038/nature05482 - DOI - PubMed
-
- Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc 2010; 69: 434–441. doi: 10.1017/S0029665110001813 - DOI - PubMed
-
- Greenwood HC, Bloom SR, Murphy KG. Peptides and their potential role in the treatment of diabetes and obesity. Rev Diabet Stud 2011; 8: 355–68. doi: 10.1900/RDS.2011.8.355 - DOI - PMC - PubMed
-
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013; 9: 13–27. doi: 10.1038/nrendo.2012.199 - DOI - PubMed
-
- Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: a favorable association in Tehranian adults. Eur J Clin Nutr 2005; 59: 353–62. doi: 10.1038/sj.ejcn.1602080 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

