Altered dopaminergic regulation of the dorsal striatum is able to induce tic-like movements in juvenile rats
- PMID: 29698507
- PMCID: PMC5919623
- DOI: 10.1371/journal.pone.0196515
Altered dopaminergic regulation of the dorsal striatum is able to induce tic-like movements in juvenile rats
Abstract
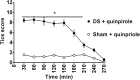
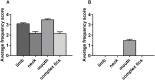
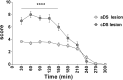
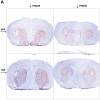
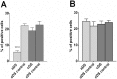
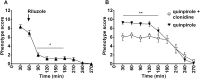
Motor tics are sudden, repetitive, involuntary movements representing the hallmark behaviors of the neurodevelopmental disease Tourette's syndrome (TS). The primary cause of TS remains unclear. The initial observation that dopaminergic antagonists alleviate tics led to the development of a dopaminergic theory of TS etiology which is supported by post mortem and in vivo studies indicating that non-physiological activation of the striatum could generate tics. The striatum controls movement execution through the balanced activity of dopamine receptor D1 and D2-expressing medium spiny neurons of the direct and indirect pathway, respectively. Different neurotransmitters can activate or repress striatal activity and among them, dopamine plays a major role. In this study we introduced a chronic dopaminergic alteration in juvenile rats, in order to modify the delicate balance between direct and indirect pathway. This manipulation was done in the dorsal striatum, that had been associated with tic-like movements generation in animal models. The results were movements resembling tics, which were categorized and scored according to a newly developed rating scale and were reduced by clonidine and riluzole treatment. Finally, post mortem analyses revealed altered RNA expression of dopaminergic receptors D1 and D2, suggesting an imbalanced dopaminergic regulation of medium spiny neuron activity as being causally related to the observed phenotype.
Conflict of interest statement
Figures




References
-
- Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: A systematic review and meta-analysis. Mov Disord. 2015;30: 221–228. doi: 10.1002/mds.26089 - DOI - PubMed
-
- Singer HS. Tourette syndrome and other tic disorders In: Weiner WJ, Tolosa E, editors. Handbook of Clinical Neurology. Elsevier; 2011. pp. 641–657. doi: 10.1016/B978-0-444-52014-2.00046-X - DOI - PubMed
-
- Robertson MM, Eapen V, Singer HS, Martino D, Scharf JM, Paschou P, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primer. 2017;3: 16097 doi: 10.1038/nrdp.2016.97 - DOI - PubMed
-
- Ganos C, Martino D. Tics and Tourette Syndrome. Neurol Clin. 2015;33: 115–136. doi: 10.1016/j.ncl.2014.09.008 - DOI - PubMed
-
- Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, et al. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60: 415–424. doi: 10.1001/archpsyc.60.4.415 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

