MicroRNA-425 and microRNA-155 cooperatively regulate atrial natriuretic peptide expression and cGMP production
- PMID: 29698509
- PMCID: PMC5919659
- DOI: 10.1371/journal.pone.0196697
MicroRNA-425 and microRNA-155 cooperatively regulate atrial natriuretic peptide expression and cGMP production
Abstract
Aims: Atrial natriuretic peptide (ANP), secreted primarily by atrial cardiomyocytes, decreases blood pressure by raising cyclic 3',5'-guanosine monophosphate (cGMP) levels and inducing vasorelaxation, natriuresis, and diuresis. Raising the level of ANP has been shown to be an effective treatment for hypertension. To advance the future development of an anti-microRNA (miR) approach to increasing expression of ANP, we investigated the regulation of NPPA expression by two miRs: miR-425 and miR-155. We examined whether miR-425 and miR-155 have an additive effect on the expression and function of ANP.
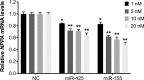
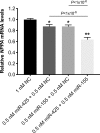
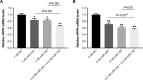
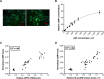
Methods and results: Human embryonic stem cell-derived cardiomyocytes (hESC-CMs) were transfected with miR-425, miR-155, or a combination of the two miRs. Two days later, NPPA expression was measured using real time qPCR. Each of the miRs decreased NPPA expression over a wide range of concentrations, with a significant reduction at concentrations as low as 1 nM. The combination of miR-425 and miR-155 reduced NPPA expression to a greater extent than either miR-425 or miR-155 alone. An in vitro assay was developed to study the potential biological significance of the miR-induced decrease in NPPA expression. The cooperative effect of miR-425 and miR-155 on NPPA expression was associated with a significant decrease in cGMP levels.
Conclusions: These data demonstrate that miR-425 and miR-155 regulate NPPA expression in a cooperative manner. Targeting both miRNAs with anti-miRs (possibly at submaximal concentrations) might prove to be a more effective strategy to modulate ANP levels, and thus blood pressure, than targeting either miRNA alone.
Conflict of interest statement
Figures
Similar articles
-
Novel MicroRNA Regulators of Atrial Natriuretic Peptide Production.Mol Cell Biol. 2016 Jun 29;36(14):1977-87. doi: 10.1128/MCB.01114-15. Print 2016 Jul 15. Mol Cell Biol. 2016. PMID: 27185878 Free PMC article.
-
Implication of microRNAs in atrial natriuretic peptide and nitric oxide signaling in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2011 Oct;301(4):C929-37. doi: 10.1152/ajpcell.00088.2011. Epub 2011 Jul 6. Am J Physiol Cell Physiol. 2011. PMID: 21734186 Free PMC article.
-
Antisense regulation of atrial natriuretic peptide expression.JCI Insight. 2019 Oct 3;4(19):e130978. doi: 10.1172/jci.insight.130978. JCI Insight. 2019. PMID: 31503546 Free PMC article.
-
Atrial natriuretic factor as a volume regulator.J Clin Pharmacol. 1994 May;34(5):424-6. doi: 10.1002/j.1552-4604.1994.tb04982.x. J Clin Pharmacol. 1994. PMID: 7916352 Review.
-
Atrial natriuretic peptide and oxidative stress.Peptides. 2010 Jul;31(7):1412-9. doi: 10.1016/j.peptides.2010.04.001. Epub 2010 Apr 10. Peptides. 2010. PMID: 20385186 Review.
Cited by
-
Biological disturbance of MiR-425 and its application prospects in cardiovascular diseases.Front Cell Dev Biol. 2025 May 9;13:1593241. doi: 10.3389/fcell.2025.1593241. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40417179 Free PMC article. Review.
-
Natriuretic peptide pathways in heart failure: further therapeutic possibilities.Cardiovasc Res. 2023 Feb 3;118(18):3416-3433. doi: 10.1093/cvr/cvac125. Cardiovasc Res. 2023. PMID: 36004816 Free PMC article.
-
MicroRNAs: The Missing Link between Hypertension and Periodontitis?Int J Mol Sci. 2024 Feb 6;25(4):1992. doi: 10.3390/ijms25041992. Int J Mol Sci. 2024. PMID: 38396672 Free PMC article. Review.
-
The Role of MicroRNAs in Cancer Biology and Therapy from a Systems Biology Perspective.Adv Exp Med Biol. 2022;1385:1-22. doi: 10.1007/978-3-031-08356-3_1. Adv Exp Med Biol. 2022. PMID: 36352209 Review.
-
The pharmaco-epigenetics of hypertension: a focus on microRNA.Mol Cell Biochem. 2024 Dec;479(12):3255-3271. doi: 10.1007/s11010-024-04947-9. Epub 2024 Feb 29. Mol Cell Biochem. 2024. PMID: 38424404 Free PMC article. Review.
References
-
- Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912 ; PubMed Central PMCID: PMCPMC4979614. - DOI - PMC - PubMed
-
- Levy D, Larson MG, Benjamin EJ, Newton-Cheh C, Wang TJ, Hwang SJ, et al. Framingham Heart Study 100K Project: genome-wide associations for blood pressure and arterial stiffness. BMC Med Genet. 2007;8 Suppl 1:S3 doi: 10.1186/1471-2350-8-S1-S3 ; PubMed Central PMCID: PMCPMC1995621. - DOI - PMC - PubMed
-
- Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63(4):698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002 . - DOI - PubMed
-
- Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–66. doi: 10.1016/S0140-6736(09)61966-8 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

