Skin thickness as a potential marker of gestational age at birth despite different fetal growth profiles: A feasibility study
- PMID: 29698511
- PMCID: PMC5919437
- DOI: 10.1371/journal.pone.0196542
Skin thickness as a potential marker of gestational age at birth despite different fetal growth profiles: A feasibility study
Abstract
Background: New methodologies to estimate gestational age (GA) at birth are demanded to face the limited access to obstetric ultrasonography and imprecision of postnatal scores. The study analyzed the correlation between neonatal skin thickness and pregnancy duration. Secondarily, it investigated the influence of fetal growth profiles on tissue layer dimensions.
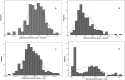
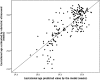
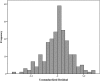
Methods and findings: In a feasibility study, 222 infants selected at a term-to-preterm ratio of 1:1 were assessed. Reliable information on GA was based on the early ultrasonography-based reference. The thicknesses of the epidermal and dermal skin layers were examined using high-frequency ultrasonography. We scanned the skin over the forearm and foot plantar surface of the newborns. A multivariate regression model was adjusted to determine the correlation of GA with skin layer dimensions. The best model to correlate skin thickness with GA was fitted using the epidermal layer on the forearm site, adjusted to cofactors, as follows: Gestational age (weeks) = -28.0 + 12.8 Ln (Thickness) - 4.4 Incubator staying; R2 = 0.604 (P<0.001). In this model, the constant value for the standard of fetal growth was statistically null. The dermal layer thickness on the forearm and plantar surfaces had a negative moderate linear correlation with GA (R = -0.370, P<0.001 and R = -0.421, P<0.001, respectively). The univariate statistical analyses revealed the influence of underweight and overweight profiles on neonatal skin thickness at birth. Of the 222 infants, 53 (23.9%) had inappropriate fetal growths expected for their GA. Epidermal thickness was not fetal growth standard dependent as follows: 172.2 (19.8) μm for adequate for GA, 171.4 (20.6) μm for SGA, and 177.7 (15.2) μm for LGA (P = 0.525, mean [SD] on the forearm).
Conclusions: The analysis highlights a new opportunity to relate GA at birth to neonatal skin layer thickness. As this parameter was not influenced by the standard of fetal growth, skin maturity can contribute to clinical applications.
Conflict of interest statement
Figures


Similar articles
-
Single and Serial Fetal Biometry to Detect Preterm and Term Small- and Large-for-Gestational-Age Neonates: A Longitudinal Cohort Study.PLoS One. 2016 Nov 1;11(11):e0164161. doi: 10.1371/journal.pone.0164161. eCollection 2016. PLoS One. 2016. PMID: 27802270 Free PMC article.
-
Greater estimated fetal weight and birth weight in IVF/ICSI pregnancy after frozen-thawed vs fresh blastocyst transfer: prospective cohort study with novel unified modeling methodology.Ultrasound Obstet Gynecol. 2022 Jul;60(1):76-85. doi: 10.1002/uog.24806. Ultrasound Obstet Gynecol. 2022. PMID: 34716733
-
Newborn skin reflection: Proof of concept for a new approach for predicting gestational age at birth. A cross-sectional study.PLoS One. 2017 Sep 20;12(9):e0184734. doi: 10.1371/journal.pone.0184734. eCollection 2017. PLoS One. 2017. PMID: 28931040 Free PMC article.
-
Influence of different methods for calculating gestational age at birth on prematurity and small for gestational age proportions: a systematic review with meta-analysis.BMC Pregnancy Childbirth. 2023 Feb 11;23(1):106. doi: 10.1186/s12884-023-05411-0. BMC Pregnancy Childbirth. 2023. PMID: 36774458 Free PMC article.
-
Skin thickness dimensions in histological section measurement during late-fetal and neonatal developmental period: A systematic review.Skin Res Technol. 2019 Nov;25(6):793-800. doi: 10.1111/srt.12719. Epub 2019 May 22. Skin Res Technol. 2019. PMID: 31119813 Free PMC article.
Cited by
-
2-kDa hyaluronan ameliorates human facial wrinkles through increased dermal collagen density related to promotion of collagen remodeling.J Cosmet Dermatol. 2023 Jan;22(1):320-327. doi: 10.1111/jocd.15097. Epub 2022 Jun 21. J Cosmet Dermatol. 2023. PMID: 35587723 Free PMC article. Clinical Trial.
-
Relationships between Skin Structure and Skin Function of Pregnant Women and Their Infants: A Prospective Cohort Study.Skin Pharmacol Physiol. 2025 Jun 18:1-11. doi: 10.1159/000546770. Online ahead of print. Skin Pharmacol Physiol. 2025. PMID: 40532692 Free PMC article.
-
Detection of skin thickness and density in healthy Chinese people by using high-frequency ultrasound.Skin Res Technol. 2023 Jan;29(1):e13219. doi: 10.1111/srt.13219. Epub 2022 Nov 4. Skin Res Technol. 2023. PMID: 36331142 Free PMC article.
-
Noninvasive transcutaneous bilirubin assessment of neonates with hyperbilirubinemia using a photon diffusion theory-based method.Biomed Opt Express. 2019 May 23;10(6):2969-2984. doi: 10.1364/BOE.10.002969. eCollection 2019 Jun 1. Biomed Opt Express. 2019. PMID: 31259067 Free PMC article.
-
Necrotizing Enterocolitis Detection in Premature Infants Using Broadband Optical Spectroscopy.J Biophotonics. 2025 Jan;18(1):e202400273. doi: 10.1002/jbio.202400273. Epub 2024 Nov 11. J Biophotonics. 2025. PMID: 39527955 Free PMC article.
References
-
- Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine 2008;26: 3197–3208. doi: 10.1016/j.vaccine.2008.03.095 - DOI - PubMed
-
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt 2013;18: 061224–061224. doi: 10.1117/1.JBO.18.6.061224 - DOI - PubMed
-
- Meyer N, Lauwers-Cances V, Lourari S, Laurent J, Konstantinou MP, Lagarde JM, et al. High-frequency ultrasonography but not 930-nm optical coherence tomography reliably evaluates melanoma thickness in vivo: a prospective validation study. Br J Dermatol 2014;171: 799–805. doi: 10.1111/bjd.13129 - DOI - PubMed
-
- Schou A, Thomsen K, Plomgaard A, Wolthers O. Methodological aspects of high-frequency ultrasound of skin in children. Skin Res Technol 2004;10: 200–206. doi: 10.1111/j.1600-0846.2004.00070.x - DOI - PubMed
-
- Tan C, Statham B, Marks R, Payne P. Skin thickness measurement by pulsed ultrasound; its reproducibility, validation and variability. Br J Dermatol 1982;106: 657–667. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

