Practical spectrophotometric assay for the dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase, a potential antibiotic target
- PMID: 29698518
- PMCID: PMC5919655
- DOI: 10.1371/journal.pone.0196010
Practical spectrophotometric assay for the dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase, a potential antibiotic target
Abstract
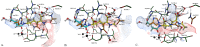
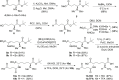
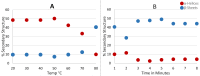
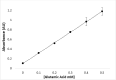
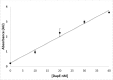
A new enzymatic assay for the bacterial enzyme succinyl-diaminopimelate desuccinylase (DapE, E.C. 3.5.1.18) is described. This assay employs N6-methyl-N2-succinyl-L,L-diaminopimelic acid (N6-methyl-L,L-SDAP) as the substrate with ninhydrin used to detect cleavage of the amide bond of the modified substrate, wherein N6-methylation enables selective detection of the primary amine enzymatic product. Molecular modeling supported preparation of the mono-N6-methylated-L,L-SDAP as an alternate substrate for the assay, given binding in the active site of DapE predicted to be comparable to the endogenous substrate. The alternate substrate for the assay, N6-methyl-L,L-SDAP, was synthesized from the tert-butyl ester of Boc-L-glutamic acid employing a Horner-Wadsworth-Emmons olefination followed by an enantioselective reduction employing Rh(I)(COD)(S,S)-Et-DuPHOS as the chiral catalyst. Validation of the new ninhydrin assay was demonstrated with known inhibitors of DapE from Haemophilus influenza (HiDapE) including captopril (IC50 = 3.4 [± 0.2] μM, 3-mercaptobenzoic acid (IC50 = 21.8 [±2.2] μM, phenylboronic acid (IC50 = 316 [± 23.6] μM, and 2-thiopheneboronic acid (IC50 = 111 [± 16] μM. Based on these data, this assay is simple and robust, and should be amenable to high-throughput screening, which is an important step forward as it opens the door to medicinal chemistry efforts toward the discovery of DapE inhibitors that can function as a new class of antibiotics.
Conflict of interest statement
Figures
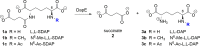
References
-
- World Health Organization. Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline, including tuberculosis. 2017. http://www.who.int/medicines/areas/rational_use/antibacterial_agents_cli...
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershrnan K, Ray S, et al. Invasive methicillin-resistant staphylococcus aureus infections in the United States. JAMA, J Am Med Assoc. 2007; 298: 1763–1771. - PubMed
-
- Howe RA, Bowker KE, Walsh TR, Feest TG, MacGowan AP. Vancomycin-resistant Staphylococcus aureus. Lancet. 1998; 351: 602 doi: 10.1016/S0140-6736(05)78597-4 - DOI - PubMed
-
- Gillner DM, Becker DP, Holz RC. Lysine biosynthesis in bacteria: a metallodesuccinylase as a potential antimicrobial target. JBIC, J Biol Inorg Chem. 2013; 18: 155–163. doi: 10.1007/s00775-012-0965-1 - DOI - PMC - PubMed
-
- Scapin G, Blanchard JS. Enzymology of bacterial lysine biosynthesis. Adv Enzymol Relat Areas Mol Biol. 1998; 72: 279–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

