Neurotoxicity of cytarabine (Ara-C) in dorsal root ganglion neurons originates from impediment of mtDNA synthesis and compromise of mitochondrial function
- PMID: 29698743
- PMCID: PMC5971160
- DOI: 10.1016/j.freeradbiomed.2018.04.570
Neurotoxicity of cytarabine (Ara-C) in dorsal root ganglion neurons originates from impediment of mtDNA synthesis and compromise of mitochondrial function
Abstract
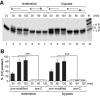
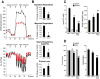
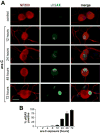
Peripheral Nervous System (PNS) neurotoxicity caused by cancer drugs hinders attainment of chemotherapy goals. Due to leakiness of the blood nerve barrier, circulating chemotherapeutic drugs reach PNS neurons and adversely affect their function. Chemotherapeutic drugs are designed to target dividing cancer cells and mechanisms underlying their toxicity in postmitotic neurons remain to be fully clarified. The objective of this work was to elucidate progression of events triggered by antimitotic drugs in postmitotic neurons. For proof of mechanism study, we chose cytarabine (ara-C), an antimetabolite used in treatment of hematological cancers. Ara-C is a cytosine analog that terminates DNA synthesis. To investigate how ara-C affects postmitotic neurons, which replicate mitochondrial but not genomic DNA, we adapted a model of Dorsal Root Ganglion (DRG) neurons. We showed that DNA polymerase γ, which is responsible for mtDNA synthesis, is inhibited by ara-C and that sublethal ara-C exposure of DRG neurons leads to reduction in mtDNA content, ROS generation, oxidative mtDNA damage formation, compromised mitochondrial respiration and diminution of NADPH and GSH stores, as well as, activation of the DNA damage response. Hence, it is plausible that in ara-C exposed DRG neurons, ROS amplified by the high mitochondrial content shifts from physiologic to pathologic levels signaling stress to the nucleus. Combined, the findings suggest that ara-C neurotoxicity in DRG neurons originates in mitochondria and that continuous mtDNA synthesis and reliance on oxidative phosphorylation for energy needs sensitize the highly metabolic neurons to injury by mtDNA synthesis terminating cancer drugs.
Keywords: Cytarabine (ara-C); DNA damage response; DNA polymerase γ; Dorsal root ganglion neurons; Mitochondria; Neurotoxicity; mtDNA.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Cisplatin Toxicity in Dorsal Root Ganglion Neurons Is Relieved by Meclizine via Diminution of Mitochondrial Compromise and Improved Clearance of DNA Damage.Mol Neurobiol. 2017 Dec;54(10):7883-7895. doi: 10.1007/s12035-016-0273-9. Epub 2016 Nov 17. Mol Neurobiol. 2017. PMID: 27858292 Free PMC article.
-
Cytosine arabinoside affects the heat and capsaicin receptor TRPV1 localisation and sensitivity in human sensory neurons.J Neurooncol. 2008 Aug;89(1):1-7. doi: 10.1007/s11060-008-9585-6. Epub 2008 Apr 15. J Neurooncol. 2008. PMID: 18414789
-
Translesion Synthesis DNA Polymerase Kappa Is Indispensable for DNA Repair Synthesis in Cisplatin Exposed Dorsal Root Ganglion Neurons.Mol Neurobiol. 2018 Mar;55(3):2506-2515. doi: 10.1007/s12035-017-0507-5. Epub 2017 Apr 8. Mol Neurobiol. 2018. PMID: 28391554 Free PMC article.
-
DNA damage response in peripheral nervous system: coping with cancer therapy-induced DNA lesions.DNA Repair (Amst). 2013 Aug;12(8):685-90. doi: 10.1016/j.dnarep.2013.04.020. Epub 2013 May 16. DNA Repair (Amst). 2013. PMID: 23684797 Free PMC article. Review.
-
[Pathways for maintenance of mitochondrial DNA integrity and mitochondrial functions in cells exposed to ionizing radiation].Radiats Biol Radioecol. 2013 Mar-Apr;53(2):117-36. doi: 10.7868/s0869803113020045. Radiats Biol Radioecol. 2013. PMID: 23786028 Review. Russian.
Cited by
-
LIS1 and NDEL1 Regulate Axonal Trafficking of Mitochondria in Mature Neurons.Front Mol Neurosci. 2022 Apr 7;15:841047. doi: 10.3389/fnmol.2022.841047. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35465088 Free PMC article.
-
Parp1 hyperactivity couples DNA breaks to aberrant neuronal calcium signalling and lethal seizures.EMBO Rep. 2021 May 5;22(5):e51851. doi: 10.15252/embr.202051851. Epub 2021 May 1. EMBO Rep. 2021. PMID: 33932076 Free PMC article.
-
Activation of the STING pathway induces peripheral sensitization via neuroinflammation in a rat model of bone cancer pain.Inflamm Res. 2023 Jan;72(1):117-132. doi: 10.1007/s00011-022-01663-2. Epub 2022 Nov 8. Inflamm Res. 2023. PMID: 36346430 Free PMC article.
-
Oxygen matters: Unraveling the role of oxygen in the neuronal response to cisplatin.J Peripher Nerv Syst. 2024 Dec;29(4):528-536. doi: 10.1111/jns.12659. Epub 2024 Sep 27. J Peripher Nerv Syst. 2024. PMID: 39329299 Free PMC article.
-
Exogenous mitochondrial transfer and endogenous mitochondrial fission facilitate AML resistance to OxPhos inhibition.Blood Adv. 2021 Oct 26;5(20):4233-4255. doi: 10.1182/bloodadvances.2020003661. Blood Adv. 2021. PMID: 34507353 Free PMC article.
References
-
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. - PubMed
-
- Beijers AJ, Jongen JL, Vreugdenhil G. Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies. Neth J Med. 2012;70:18–25. - PubMed
-
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. - PubMed
-
- Grant S. Ara-C: cellular and molecular pharmacology. Adv Cancer Res. 1998;72:197–233. - PubMed
-
- Iliakis G, Bryant PE. Effects of the nucleoside analogues alpha-ara A, beta-ara A and beta-ara C on cell growth and repair of both potentially lethal damage and DNA double strand breaks in mammalian cells in culture. Anticancer Res. 1983;3:143–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

