Biological correlates of self-reported new and continued abstinence in cannabis cessation treatment clinical trials
- PMID: 29698894
- PMCID: PMC5959795
- DOI: 10.1016/j.drugalcdep.2018.03.017
Biological correlates of self-reported new and continued abstinence in cannabis cessation treatment clinical trials
Abstract
Background: The agreement between self-reported cannabis abstinence with urine cannabinoid concentrations in a clinical trials setting is not well characterized. We assessed the agreement between various cannabinoid cutoffs and self-reported abstinence across three clinical trials, one including contingency management for abstinence.
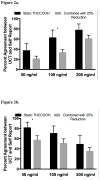
Methods: Three cannabis cessation clinical trials where participants reported use and provided weekly urine samples for cannabis and creatinine concentration measurements were included. Bootstrapped data were assessed for agreement between self-reported 7+ day abstinence and urine cannabinoid tests using generalized linear mixed effects models for clustered binary outcomes. One study implemented contingency management for cannabis abstinence. Four hundred and seventy-three participants with 3787 valid urine specimens were included. Urine was analyzed for 11-nor-9-carboxy-Δ9-tetrahydrocannabinol and creatinine using immunoassay methods Biological cutoffs of 50, 100, and 200 ng/ml, as well as changes in CN normalized THCCOOH (25%/50% decrease), were assessed for agreement with self-reported abstinence during the three clinical trials.
Results: Agreement between measured THCCOOH and self-reported abstinence increases with increasing cutoff concentrations, while the agreement with self-reported non-abstinence decreases with increasing cutoff concentrations. Combining THCCOOH cutoffs with recent changes in CN-THCCOOH provides a better agreement in those self-reporting abstinence. Participants in the studies that received CM for abstinence had a lower agreement between self-reported abstinence and returned to use than those in studies that did not have a contingency management component.
Conclusion: Using combinations of biological measurements and self-reported abstinence, confirmation of study related abstinence may be verifiable earlier and with greater accuracy than relying on a single measurement.
Keywords: Cannabis; Clinical trials; Concentrations; Contingency management; Self-Report; Urine cannabinoid.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
No conflict declared.
Figures

References
-
- Carey KB. Reliability and validity of the time-line follow-back interview among psychiatric outpatients: A preliminary report. Psychol Addict Behav. 1997;11:26–33.
-
- Cheng G, Yu Z, Huang JZ. The cluster bootstrap consistency in generalized estimating equations. J Multivar Anal. 2013;115:33–47.
-
- Dackis CA, Pottash AIC, Annitto W, Gold MS. Persistence of urinary marijuana levels after supervised abstinence. Am J Addict. 1982;139:1196–1198. - PubMed
-
- Del Boca FK, Noll JA. Truth or consequences: The validity of self-report data in health services research on addictions. Addiction. 2000;95:S347–60. - PubMed
-
- Donovan DM, Bigelow GE, Brigham GS, Carrol KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wakin PG, Wells EA. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107:694–708. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

