Regulation of hyperactivation of hamster spermatozoa by progesterone
- PMID: 29699287
- PMCID: PMC5904637
- DOI: 10.1111/j.1447-0578.2008.00202.x
Regulation of hyperactivation of hamster spermatozoa by progesterone
Abstract
Aim: Although it is accepted that progesterone (P) induces acrosome reaction through non-genomic regulation, it is not well known if P also affects hyperactivation of sperm. Methods: Hamster spermatozoa were hyperactivated by incubation for 4 h on modified Tyrode's albumin lactate pyruvate medium and recorded on a DVD via a charge-coupled device camera attached to a microscope with phase-contrast illumination and a small CO2 incubator. Phosphorylation of proteins was detected by western blotting using antiphosphotyrosine antibodies. Results: Sperm hyperactivation was significantly increased and accelerated by a non-genomic signal of P. Although acceleration of motility of hyperactivated sperm occurred with 10, 20 and 40 ng/mL P, the most effective concentration was 20 ng/mL. Progesterone also significantly increased 80-kDa tyrosine phosphorylation of sperm proteins. Both extracellular Ca2+ and albumin were essential for sperm hyperactivation, and the former was also essential for maintaining sperm flagellar movement. Moreover, phospholipase C (PLC) was associated with the regulation of hyperactivation by P. Conclusion: It is likely that P regulates sperm hyperactivation by a non-genomic signal in relation to tyrosine phosphorylation and PLC. (Reprod Med Biol 2008; 7: 63-74).
Keywords: capacitation; hyperactivation; non‐genomic regulation; progesterone; spermatozoa.
Figures
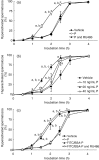
 ), mTALP + 20 ng/mL P + 0.1% EtOH (P;
), mTALP + 20 ng/mL P + 0.1% EtOH (P;  ) and mTALP + 20 ng/mL P + 23.4 µmol/L RU486 + 0.1% EtOH (P and RU486,
) and mTALP + 20 ng/mL P + 23.4 µmol/L RU486 + 0.1% EtOH (P and RU486,  ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P and RU486 (P < 0.01). (b) Sperm hyperactivation significantly increased by P in a concentration‐dependent manner. Values are means ± standard deviation. Vehicle (
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P and RU486 (P < 0.01). (b) Sperm hyperactivation significantly increased by P in a concentration‐dependent manner. Values are means ± standard deviation. Vehicle ( ), 10 ng/mL P (
), 10 ng/mL P ( ), 20 ng/mL P (
), 20 ng/mL P ( ) and 40 ng/mL P (
) and 40 ng/mL P ( ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from 10 ng/mL P (P < 0.01); csignificantly different from 40 ng/mL P (P < 0.01); dsignificantly different from vehicle (P < 0.05). (c) Sperm hyperactivation increased by non‐genomic signals of P. Values are means ± standard deviation. Vehicle (
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from 10 ng/mL P (P < 0.01); csignificantly different from 40 ng/mL P (P < 0.01); dsignificantly different from vehicle (P < 0.05). (c) Sperm hyperactivation increased by non‐genomic signals of P. Values are means ± standard deviation. Vehicle ( ), 20 ng/mL P (
), 20 ng/mL P ( ), mTALP + 7 nmol/L fluorescein isothiocyanate and bovine serum albumin conjugated progesterone + 0.1% EtOH (FITC/BSA‐P,
), mTALP + 7 nmol/L fluorescein isothiocyanate and bovine serum albumin conjugated progesterone + 0.1% EtOH (FITC/BSA‐P,  ) and mTALP + 7 nmol/L FITC/BSA‐P + 23.4 µmol/L RU486 + 0.1% EtOH (FITC/BSA‐P and RU486,
) and mTALP + 7 nmol/L FITC/BSA‐P + 23.4 µmol/L RU486 + 0.1% EtOH (FITC/BSA‐P and RU486,  ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from FITC/BSA‐P and RU486 (P < 0.01); csignificantly different from FITC/BSA‐P and RU486 (P < 0.05). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from FITC/BSA‐P and RU486 (P < 0.01); csignificantly different from FITC/BSA‐P and RU486 (P < 0.05). Experiments were carried out four times using four hamsters.
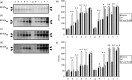

 ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), modified Tyrode's albumin lactate pyruvate (mTALP) without Ca2+ and with added 1 mmol/L ethyleneglycol bis(2‐aminoethyl ether)tetraacetic acid (EGTA) + 0.1% EtOH (Ca2+(–),
), modified Tyrode's albumin lactate pyruvate (mTALP) without Ca2+ and with added 1 mmol/L ethyleneglycol bis(2‐aminoethyl ether)tetraacetic acid (EGTA) + 0.1% EtOH (Ca2+(–),  ), mTALP without Ca2+ and with added 20 ng/mL P + 1 mmol/L EGTA + 0.1% EtOH (Ca2+(–) and P,
), mTALP without Ca2+ and with added 20 ng/mL P + 1 mmol/L EGTA + 0.1% EtOH (Ca2+(–) and P,  ). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P (P < 0.01); csignificantly different from vehicle (P < 0.05); dsignificantly different from P (P < 0.05). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle (P < 0.01); bsignificantly different from P (P < 0.01); csignificantly different from vehicle (P < 0.05); dsignificantly different from P (P < 0.05). Experiments were carried out four times using four hamsters.
 ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), modified Tyrode's albumin lactate pyruvate (mTALP) without bovine serum albumin (BSA) and with added 0.1% ethanol (EtOH) (BSA(–),
), modified Tyrode's albumin lactate pyruvate (mTALP) without bovine serum albumin (BSA) and with added 0.1% ethanol (EtOH) (BSA(–),  ) and mTALP without BSA and with added 20 ng/mL P + 0.1% EtOH (BSA(–) and P,
) and mTALP without BSA and with added 20 ng/mL P + 0.1% EtOH (BSA(–) and P,  ). aSignificantly different from vehicle (P < 0.05); bsignificantly different from P (P < 0.05); csignificantly different from vehicle (P < 0.01); dsignificantly different from P (P < 0.01). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle (P < 0.05); bsignificantly different from P (P < 0.05); csignificantly different from vehicle (P < 0.01); dsignificantly different from P (P < 0.01). Experiments were carried out four times using four hamsters.
 ), 1 µmol/L U73122 (U73122,
), 1 µmol/L U73122 (U73122,  ), 1 µmol/L U73343 (U73343,
), 1 µmol/L U73343 (U73343,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), 20 ng/mL P + 1 µmol/L U73122 (P and U73122,
), 20 ng/mL P + 1 µmol/L U73122 (P and U73122,  ) and 20 ng/mL P + 1 µmol/L U73343 (P and U73343,
) and 20 ng/mL P + 1 µmol/L U73343 (P and U73343,  ). (b) D609, which is an inhibitor of phosphatidylcholine‐PLC (PC‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (b) D609, which is an inhibitor of phosphatidylcholine‐PLC (PC‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 10 µmol/L D609 (D609,
), 10 µmol/L D609 (D609,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ), 20 ng/mL P + 10 µmol/L D609 (P and D609,
), 20 ng/mL P + 10 µmol/L D609 (P and D609,  ). (c) ET‐18‐OCH3, which is an inhibitor of phosphatidylinositol‐PLC (PI‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (c) ET‐18‐OCH3, which is an inhibitor of phosphatidylinositol‐PLC (PI‐PLC), was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 15 µmol/L ET‐18‐OCH3 (ET‐18‐OCH3,
), 15 µmol/L ET‐18‐OCH3 (ET‐18‐OCH3,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ) and 20 ng/mL P + 15 µmol/L ET‐18‐OCH3 (P and ET‐18‐OCH3,
) and 20 ng/mL P + 15 µmol/L ET‐18‐OCH3 (P and ET‐18‐OCH3,  ). (d) Neomycine, which is a non‐specific inhibitor of PLC, was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (d) Neomycine, which is a non‐specific inhibitor of PLC, was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 65 µmol/L neomycine (Neomycine,
), 65 µmol/L neomycine (Neomycine,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ) and 20 ng/mL P + 65 µmol/L neomycine (P and Neomycine,
) and 20 ng/mL P + 65 µmol/L neomycine (P and Neomycine,  ). (e) Spermine, which is an inhibitor of PLCα and activator of PLCδ, was added to the mTALP medium. Values are means ± standard deviation. Vehicle (
). (e) Spermine, which is an inhibitor of PLCα and activator of PLCδ, was added to the mTALP medium. Values are means ± standard deviation. Vehicle ( ), 1 mmol/L spermine (Spermine,
), 1 mmol/L spermine (Spermine,  ), 20 ng/mL P (P,
), 20 ng/mL P (P,  ) and 20 ng/mL P + 1 mmol/L spermine (P and Spermine,
) and 20 ng/mL P + 1 mmol/L spermine (P and Spermine,  ). aSignificantly different from vehicle and inhibitor (P < 0.01); bsignificantly different from P and inhibitor (P < 0.01); csignificantly different from vehicle and inhibitor (P < 0.05); dsignificantly different from P and inhibitor (P < 0.05). Experiments were carried out four times using four hamsters.
). aSignificantly different from vehicle and inhibitor (P < 0.01); bsignificantly different from P and inhibitor (P < 0.01); csignificantly different from vehicle and inhibitor (P < 0.05); dsignificantly different from P and inhibitor (P < 0.05). Experiments were carried out four times using four hamsters.
References
-
- Yudin AI, Gottlieb W, Meizel S. Ultrastructural studies of the early events of the human sperm acrosome reaction as initiated by human follicular fluid. Gamete Res 1988; 20: 11–24. - PubMed
-
- Yanagimachi R. Mammalian fertilization In: Neill K, Pfaff GM, eds. The Physiology of Reproduction, Vol. 1, 2nd edn. New York: Raven Press, 1994; 189–317.
-
- Morisawa M. Cell signaling mechanisms for sperm motility. Zool Sci 1994; 11: 647–662. - PubMed
-
- Suarez SS, Ho HC. Hyperactivated motility in sperm. Reprod Dom Anim 2003; 38: 119–124. - PubMed
-
- Langlais J, Roberts KD. A molecular membrane model of sperm capacitation and the acrosome reaction of mammalian spermatozoa. Gamete Res 1985; 13: 183–224.
LinkOut - more resources
Full Text Sources
Miscellaneous
