The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study
- PMID: 29699509
- PMCID: PMC5921787
- DOI: 10.1186/s12885-018-4353-2
The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study
Abstract
Background: This pilot trial aimed to study the feasibility and effects on quality of life (QOL) and well-being of short-term fasting (STF) during chemotherapy in patients with gynecological cancer.
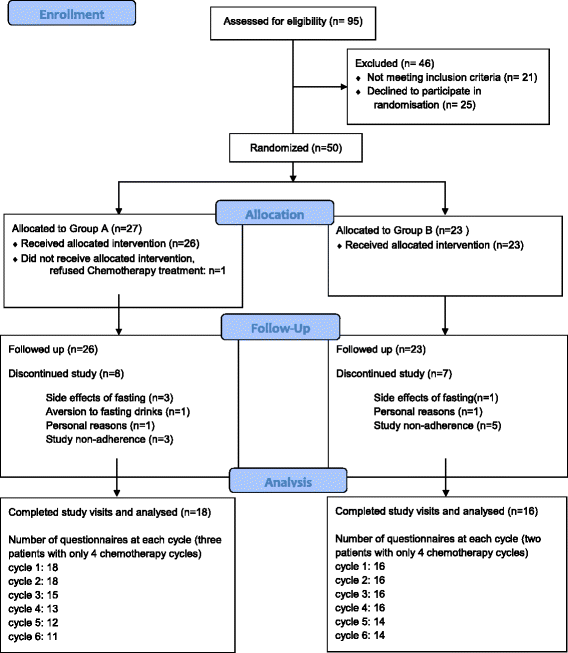
Methods: In an individually-randomized cross-over trial patients with gynecological cancer, 4 to 6 planned chemotherapy cycles were included. Thirty-four patients were randomized to STF in the first half of chemotherapies followed by normocaloric diet (group A;n = 18) or vice versa (group B;n = 16). Fasting started 36 h before and ended 24 h after chemotherapy (60 h-fasting period). QOL was assessed by the FACIT-measurement system.
Results: The chemotherapy-induced reduction of QOL was less than the Minimally Important Difference (MID; FACT-G = 5) with STF but greater than the MID for non-fasted periods. The mean chemotherapy-induced deterioration of total FACIT-F was 10.4 ± 5.3 for fasted and 27.0 ± 6.3 for non-fasted cycles in group A and 14.1 ± 5.6 for non-fasted and 11.0 ± 5.6 for fasted cycles in group B. There were no serious adverse effects.
Conclusion: STF during chemotherapy is well tolerated and appears to improve QOL and fatigue during chemotherapy. Larger studies should prove the effect of STF as an adjunct to chemotherapy.
Trial registration: This trial was registered at clinicaltrials.gov: NCT01954836 .
Keywords: Breast cancer; Chemotherapy; Fasting; Ovarian cancer; Pilot study; Quality of life.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was reviewed and approved by the Ethics Committee of the Charité-University Medical Center, Berlin EA4/088/13. All study participants gave their written informed consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S, Merlino A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra127. doi: 10.1126/scitranslmed.3003293. - DOI - PMC - PubMed
-
- Tannenbaum A, Silverstone H. The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer Res. 1949;9(12):724–727. - PubMed
-
- Michalsen A, Li C, Kaiser K, Lüdtke R, Meier L, Stange R, Kessler C. In-patient treatment of fibromyalgia: a controlled nonrandomized comparison of conventional medicine versus integrative medicine including fasting therapy. Evid Based Complement Alternat Med. 2013;2013:908610. doi: 10.1155/2013/908610. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

