Evaluating clinical effectiveness of 13-valent pneumococcal conjugate vaccination against pneumonia among middle-aged and older adults in Catalonia: results from the EPIVAC cohort study
- PMID: 29699550
- PMCID: PMC5921259
- DOI: 10.1186/s12879-018-3096-7
Evaluating clinical effectiveness of 13-valent pneumococcal conjugate vaccination against pneumonia among middle-aged and older adults in Catalonia: results from the EPIVAC cohort study
Abstract
Background: Benefits using the 13-valent pneumococcal conjugate vaccine (PCV13) in adults are controversial. This study investigated clinical effectiveness of PCV13 vaccination in preventing hospitalisation from pneumonia among middle-aged and older adults.
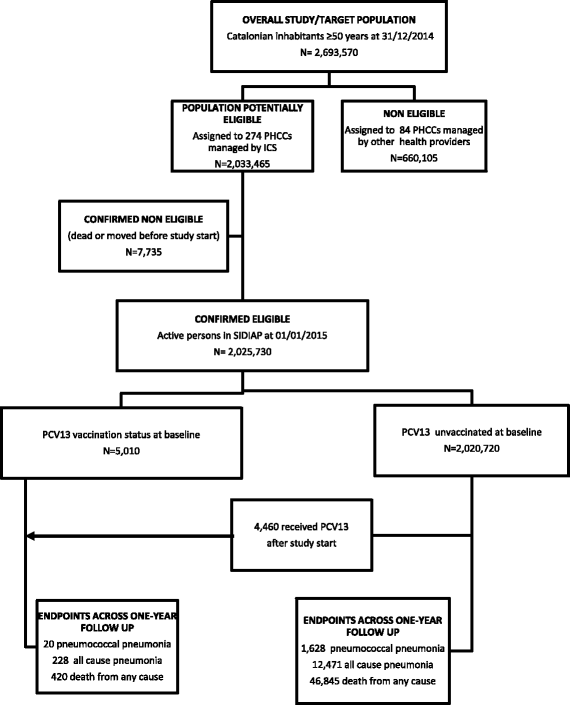
Methods: Population-based cohort study involving 2,025,730 individuals ≥50 years in Catalonia, Spain, who were prospectively followed from 01/01/2015 to 31/12/2015. Primary outcomes were hospitalisation for pneumococcal or all-cause pneumonia and death from any cause. Cox regression models were used to evaluate the association between PCV13 vaccination and the risk of each outcome, adjusting for age, sex and major comorbidities/underlying risk conditions.
Results: Cohort members were observed for a total of 1,990,701 person-years, of which 6912 person-years were PCV13 vaccinated. Overall, crude incidence rates (per 100,000 person-years) were 82.8 (95% confidence interval [CI]: 77.7-88.1) for pneumococcal pneumonia, 637.9 (95% CI: 599.0-678.7) for all-cause pneumonia and 2367.2 (95% CI: 2222.8-2518.7) for all-cause death. After multivariable adjustments we found that the PCV13 vaccination did not alter significantly the risk of pneumococcal pneumonia (multivariable-adjusted hazard ratio [mHR]: 1.17; 95% CI: 0.75-1.83; p = 0.493) and all-cause death (mHR: 1.07; 95% CI: 0.97-1.18; p = 0.190), although it remained significantly associated with an increased risk of all-cause pneumonia (mHR: 1.69; 95% CI: 1.48-1.94; p < 0.001). In stratified analyses focused on middle-aged or elderly persons and immunocompromised or immunocompetent subjects, PCV13 vaccination did not appear effective either.
Conclusion: Our data does not support clinical benefits of PCV13 vaccination against pneumonia among adults in Catalonia. It must be closely monitored in future studies involving more vaccinated person-time at-observation.
Keywords: Adults; Effectiveness; Pneumococcal conjugate vaccine; Pneumonia; Streptococcus pneumoniae.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the ethical committee of the Institution (ethic committee IDIAP Jordi Gol P14/134) and was conducted in accordance with the general principles for observational studies. Given this is a non-interventional study, an informed consent for all 2,025,730 study participants was formally waived by the ethics committee and was not required. Data were anonymized and risk of identification was null.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Jackson LA, Neuzil KM. Pneumococcal polysaccharide vaccines, Ch 24. In: Vaccines. Plotkin SA, Orenstein WA, Offit PA (Eds.), 5th edn, Saunders Elsevier, Philadelphia, PA, USA, 569–604 (2008).
-
- Isturiz RE, Schmoele-Thoma B, Scott DA, Scott DA, Jodar L, Webber C, et al. Pneumococcal conjugate vaccine use in adults. Expert Rev Vaccines. 2016;15:279–293. - PubMed
-
- Centers for Disease Control and Prevention Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the advisory committee on immunization practices (ACIP) MMWR Morb Mortal Wkly Rep. 2012;61:816–819. - PubMed
-
- Centers for Disease Control and Prevention Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the advisory committee on immunization practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822–825. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources