Topically Applied Curcumin-Loaded Nanoparticles Treat Erectile Dysfunction in a Rat Model of Type-2 Diabetes
- PMID: 29699754
- PMCID: PMC8826927
- DOI: 10.1016/j.jsxm.2018.03.009
Topically Applied Curcumin-Loaded Nanoparticles Treat Erectile Dysfunction in a Rat Model of Type-2 Diabetes
Abstract
Background: Curcumin, a naturally occurring anti-inflammatory compound, has shown promise in pre-clinical studies to treat erectile dysfunction (ED) associated with type-1 diabetes. However, poor bioavailability following oral administration limits its efficacy. The present study evaluated the potential of topical application of curcumin-loaded nanoparticles (curc-np) to treat ED in a rat model of type-2 diabetes (T2D).
Aim: Determine if topical application of curc-np treats ED in a T2D rat model and modulates expression of inflammatory markers.
Methods: Curc-np (4 mg curcumin) or blank nanoparticles were applied every 2 days for 2 weeks to the shaved abdomen of 20-week-old Zucker diabetic fatty male rats (N = 5 per group). Lean Zucker diabetic fatty male rat controls were treated with blank nanoparticles (N = 5). Penetration of nanoparticles and curcumin release were confirmed by 2-photon fluorescence microscopy and histology. Erectile function was determined by measuring intracorporal pressure (ICP) normalized to systemic blood pressure (ICP/BP) following cavernous nerve stimulation. Corporal tissue was excised and reverse transcription and quantitative polymerase chain reaction used to determine expression of the following markers: nuclear factor (NF)-κβ, NF-κβ-activating protein (Nkap), NF erythroid 2-related factor-2, Kelch-like enoyl-CoA hydratase-associated protein-1, heme oxygenase-1 (HO-1), variable coding sequence-A1, phosphodiesterase-5, endothelial and neuronal nitric oxide synthase, Ras homolog gene family member A, and Rho-associated coiled-coil containing protein kinases-1 and -2.
Outcomes: Erectile function by determination of ICP/BP and expression of molecular markers in corporal tissue by RT-qPCR.
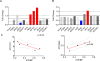
Results: Nanoparticles penetrated the abdominal epidermis and persisted in hair follicles for 24 hours. Curc-np-treated animals exhibited higher average ICP/BP than animals treated with blank nanoparticles at all levels of stimulation and this was statistically significant (P < .05) at 0.75 mA. In corporal tissue, Nkap expression decreased 60% and heme oxygenase-1 expression increased 60% in curc-np- compared to blank nanoparticle-treated animals. ICP/BP values inversely correlated with Nkap and directly correlated with HO-1 expression levels.
Clinical translation: These studies demonstrate the potential for topical application of curc-np as a treatment for ED in T2D patients.
Conclusions: The T2D animal model of ED represents a more prevalent disease than the more commonly studied type-1 diabetes model. Although there is improved erectile response in curc-np-treated animals, only at the lower levels of stimulation (0.75 mA) was this significant compared to the blank nanoparticle-treated animals, suggesting more studies are needed to optimize protocols and evaluate toxicity. Topical application of curc-np to a rat model of T2D can systemically deliver curcumin, treat ED, and modulate corporal expression of inflammatory markers. Draganski A, Tar MT, Villegas G, et al. Topically Applied Curcumin-Loaded Nanoparticles Treat Erectile Dysfunction in a Rat Model of Type-2 Diabetes. J Sex Med 2018;15:645-653.
Keywords: Curcumin; Diabetes; Erectile Dysfunction; Inflammation; Nanoparticle.
Copyright © 2018 International Society for Sexual Medicine. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421–6. - PubMed
-
- Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151:765–9. - PubMed
-
- Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med. 2009;6(Suppl 3):254–61. - PubMed
-
- Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–75. - PubMed
-
- Santi D, Granata AR, Guidi A, et al. Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: a randomized, double-blind, prospective trial. Eur J Endocrinol. 2016;174:513–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

