Opportunities and Challenges in Cardiovascular Pharmacogenomics: From Discovery to Implementation
- PMID: 29700066
- PMCID: PMC5926807
- DOI: 10.1161/CIRCRESAHA.117.310965
Opportunities and Challenges in Cardiovascular Pharmacogenomics: From Discovery to Implementation
Abstract
This review will provide an overview of the principles of pharmacogenomics from basic discovery to implementation, encompassing application of tools of contemporary genome science to the field (including areas of apparent divergence from disease-based genomics), a summary of lessons learned from the extensively studied drugs clopidogrel and warfarin, the current status of implementing pharmacogenetic testing in practice, the role of genomics and related tools in the drug development process, and a summary of future opportunities and challenges.
Keywords: clopidogrel; genomics; pharmacogenetics; pharmacokinetics; warfarin.
© 2018 American Heart Association, Inc.
Figures
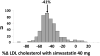

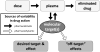



References
-
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360. - PubMed
-
- Pirmohamed M. Personalized pharmacogenomics: Predicting efficacy and adverse drug reactions. Annual review of genomics and human genetics. 2014;15:349–370. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. Journal of the American Medical Association. 1998;279:1200–1205. - PubMed
-
- Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. New England Journal of Medicine. 2010;363:2124–2134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

