In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss
- PMID: 29700102
- PMCID: PMC6003767
- DOI: 10.1183/13993003.01680-2017
In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss
Abstract
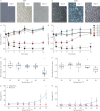
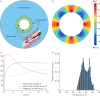
Bronchial thermoplasty is a treatment for asthma. It is currently unclear whether its histopathological impact is sufficiently explained by the proportion of airway wall that is exposed to temperatures necessary to affect cell survival.Airway smooth muscle and bronchial epithelial cells were exposed to media (37-70°C) for 10 s to mimic thermoplasty. In silico we developed a mathematical model of airway heat distribution post-thermoplasty. In vivo we determined airway smooth muscle mass and epithelial integrity pre- and post-thermoplasty in 14 patients with severe asthma.In vitro airway smooth muscle and epithelial cell number decreased significantly following the addition of media heated to ≥65°C. In silico simulations showed a heterogeneous heat distribution that was amplified in larger airways, with <10% of the airway wall heated to >60°C in airways with an inner radius of ∼4 mm. In vivo at 6 weeks post-thermoplasty, there was an improvement in asthma control (measured via Asthma Control Questionnaire-6; mean difference 0.7, 95% CI 0.1-1.3; p=0.03), airway smooth muscle mass decreased (absolute median reduction 5%, interquartile range (IQR) 0-10; p=0.03) and epithelial integrity increased (14%, IQR 6-29; p=0.007). Neither of the latter two outcomes was related to improved asthma control.Integrated in vitro and in silico modelling suggest that the reduction in airway smooth muscle post-thermoplasty cannot be fully explained by acute heating, and nor did this reduction confer a greater improvement in asthma control.
Copyright ©ERS 2018.
Conflict of interest statement
Conflict of interest: R.M. Saunders reports grants from 7th EU Framework, Wellcome Trust and National Institute for Health Research, during the conduct of the study. Conflict of interest: A.H. Mansur has received an educational grant for service support from AstraZeneca Pharmaceuticals, and received fees for talks and advisory group contribution and conference attendance from Novartis, GlaxoSmithKline, AstraZeneca, Napp Pharmaceuticals, Boehringer Ingelheim and others, outside the submitted work. Conflict of interest: P.H. Howarth reports grants from the European Union (AirPROM collaborative grant), during the conduct of the study. Conflict of interest: R. Chaudhuri reports being an advisory board member for GlaxoSmithKline, AstraZeneca, Teva Pharmaceutical Industries and Novartis and receiving educational grants for her institute from Novartis; receiving fees for speaking at meetings organised by GlaxoSmithKline, AstraZeneca, Chiesi and for attending international conferences sponsored by Novartis, Teva Pharmaceutical Industries, AstraZeneca and Boehringer Ingelheim. Conflict of interest: S. Siddiqui reports personal fees for advisory board participation from AstraZeneca and Boehringer Ingelheim, personal fees for advisory/consulting from Owlstone Nanotech and Mundipharma, speaker fees from Novartis, grants for imaging research in asthma from Napp Pharmaceuticals, and speaker fees from the European Respiratory Society, outside the submitted work. Conflict of interest: C.E. Brightling has received, paid to his institution, grants and consultancy fees from GlaxoSmithKline, Novartis, Chiesi, MedImmune/AstraZeneca, Boehringer Ingelheim, MSD Pharmaceuticals, PrEP Biopharm, Vectura, Teva Pharmaceutical Industries, Sanofi, Regeneron and Roche/Genentech. Conflict of interest: I.L. Chernyavsky reports research fellowship support from the European Commission (FP7 AirPROM) and a grant from Medical Research Council UK (New Investigator Research Grant), during the conduct of the study.
Figures


References
-
- US FDA. Alair Bronchial Thermoplasty System. Summary of safety and effectiveness data. 2010 www.accessdata.fda.gov/cdrh_docs/pdf8/P080032b.pdf Date last accessed: April 24, 2018.
-
- Wilhelm CP, Chipps BE. Bronchial thermoplasty: a review of the evidence. Ann Allergy Asthma Immunol 2016; 116: 92–98. - PubMed
-
- Seow CY, Fredberg JJ. Historical perspective on airway smooth muscle: the saga of a frustrated cell. J Appl Physiol 2001; 91: 938–952. - PubMed
-
- Mitzner W. Airway smooth muscle: the appendix of the lung. Am J Respir Crit Care Med 2004; 169: 787–790. - PubMed
-
- Zuyderduyn S, Sukkar MB, Fust A, et al. . Treating asthma means treating airway smooth muscle cells. Eur Respir J 2008; 32: 265–274. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
