COX-2 expression mediated by calcium-TonEBP signaling axis under hyperosmotic conditions serves osmoprotective function in nucleus pulposus cells
- PMID: 29700115
- PMCID: PMC5995512
- DOI: 10.1074/jbc.RA117.001167
COX-2 expression mediated by calcium-TonEBP signaling axis under hyperosmotic conditions serves osmoprotective function in nucleus pulposus cells
Abstract
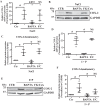
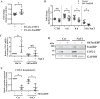
The nucleus pulposus (NP) of intervertebral discs experiences dynamic changes in tissue osmolarity because of diurnal loading of the spine. TonEBP/NFAT5 is a transcription factor that is critical in osmoregulation as well as survival of NP cells in the hyperosmotic milieu. The goal of this study was to investigate whether cyclooxygenase-2 (COX-2) expression is osmoresponsive and dependent on TonEBP, and whether it serves an osmoprotective role. NP cells up-regulated COX-2 expression in hyperosmotic media. The induction of COX-2 depended on elevation of intracellular calcium levels and p38 MAPK pathway, but independent of calcineurin signaling as well as MEK/ERK and JNK pathways. Under hyperosmotic conditions, both COX-2 mRNA stability and its proximal promoter activity were increased. The proximal COX-2 promoter (-1840/+123 bp) contained predicted binding sites for TonEBP, AP-1, NF-κB, and C/EBP-β. While COX-2 promoter activity was positively regulated by both AP-1 and NF-κB, AP-1 had no effect and NF-κB negatively regulated COX-2 protein levels under hyperosmotic conditions. On the other hand, TonEBP was necessary for both COX-2 promoter activity and protein up-regulation in response to hyperosmotic stimuli. Ex vivo disc organ culture studies using hypomorphic TonEBP+/- mice confirmed that TonEBP is required for hyperosmotic induction of COX-2. Importantly, the inhibition of COX-2 activity under hyperosmotic conditions resulted in decreased cell viability, suggesting that COX-2 plays a cytoprotective and homeostatic role in NP cells for their adaptation to dynamically loaded hyperosmotic niches.
Keywords: COX-2; NFAT transcription factor; TonEBP; calcium; cell biology; cell signaling; cyclooxygenase (COX); intervertebral disc; nucleus pulposus; osmoregulation; transcription factor.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

