The early expansion of anergic NKG2Apos/CD56dim/CD16neg natural killer represents a therapeutic target in haploidentical hematopoietic stem cell transplantation
- PMID: 29700172
- PMCID: PMC6068034
- DOI: 10.3324/haematol.2017.186619
The early expansion of anergic NKG2Apos/CD56dim/CD16neg natural killer represents a therapeutic target in haploidentical hematopoietic stem cell transplantation
Abstract
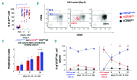
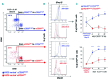
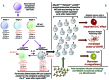
Natural killer cells are the first lymphocyte population to reconstitute early after non-myeloablative and T cell-replete haploidentical hematopoietic stem cell transplantation with post-transplant infusion of cyclophosphamide. The study herein characterizes the transient and predominant expansion starting from the second week following haploidentical hematopoietic stem cell transplantation of a donor-derived unconventional subset of NKp46neg-low/CD56dim/CD16neg natural killer cells expressing remarkably high levels of CD94/NKG2A. Both transcription and phenotypic profiles indicated that unconventional NKp46neg-low/CD56dim/CD16neg cells are a distinct natural killer cell subpopulation with features of late stage differentiation, yet retaining proliferative capability and functional plasticity to generate conventional NKp46pos/CD56bright/CD16neg-low cells in response to interleukin-15 plus interleukin-18. While present at low frequency in healthy donors, unconventional NKp46neg-low/CD56dim/CD16neg cells are greatly expanded in the seven weeks following haploidentical hematopoietic stem cell transplantation, and express high levels of the activating receptors NKG2D and NKp30 as well as of the lytic granules Granzyme-B and Perforin. Nonetheless, NKp46neg-low/CD56dim/CD16neg cells displayed a markedly defective cytotoxicity that could be reversed by blocking the inhibitory receptor CD94/NKG2A. These data open new and important perspectives to better understand the ontogenesis/homeostasis of human natural killer cells and to develop a novel immune-therapeutic approach that targets the inhibitory NKG2A check-point, thus unleashing natural killer cell alloreactivity early after haploidentical hematopoietic stem cell transplantation.
Trial registration: ClinicalTrials.gov NCT02459301.
Copyright© 2018 Ferrata Storti Foundation.
Figures





References
-
- Patriarca F, Luznik L, Medeot M, et al. Experts’ considerations on HLA-haploidentical stem cell transplantation. Eur J Haematology. 2014;93(3):187–197. - PubMed
-
- Castagna L, Crocchiolo R, Furst S, et al. Bone marrow compared with peripheral blood stem cells for haploidentical transplantation with a nonmyeloablative conditioning regimen and post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2014;20(5):724–729. - PubMed
-
- Imamura M, Tanaka J. Immunoregulatory cells for transplantation tolerance and graft-versus-leukemia effect. Int J Hematol. 2003;78(3):188–194. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

