The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution
- PMID: 29700227
- PMCID: PMC6038144
- DOI: 10.1126/science.aar5780
The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution
Abstract
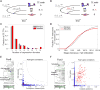
Time series of single-cell transcriptome measurements can reveal dynamic features of cell differentiation pathways. From measurements of whole frog embryos spanning zygotic genome activation through early organogenesis, we derived a detailed catalog of cell states in vertebrate development and a map of differentiation across all lineages over time. The inferred map recapitulates most if not all developmental relationships and associates new regulators and marker genes with each cell state. We find that many embryonic cell states appear earlier than previously appreciated. We also assess conflicting models of neural crest development. Incorporating a matched time series of zebrafish development from a companion paper, we reveal conserved and divergent features of vertebrate early developmental gene expression programs.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests: A.M.K. and M.W.K are founders of 1Cell-Bio, Inc. All other authors declare no competing interests.
Figures
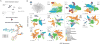





Comment in
-
A new view of embryo development and regeneration.Science. 2018 Jun 1;360(6392):967-968. doi: 10.1126/science.aat8413. Science. 2018. PMID: 29853675 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

