Class I histone deacetylase (HDAC) inhibitor CI-994 promotes functional recovery following spinal cord injury
- PMID: 29700327
- PMCID: PMC5919919
- DOI: 10.1038/s41419-018-0543-8
Class I histone deacetylase (HDAC) inhibitor CI-994 promotes functional recovery following spinal cord injury
Abstract
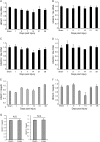
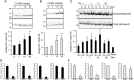
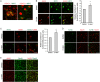
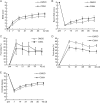
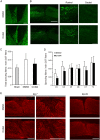
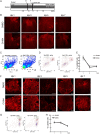
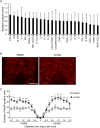
Spinal cord injury (SCI) induces severe and long-lasting neurological disability. Accumulating evidence has suggested that histone deacetylase (HDAC) inhibitors exert neuroprotective effects against various insults and deficits in the central nervous system. In the present study, we assessed the effect of the class I HDAC inhibitor CI-994 in a mouse model of SCI. Following SCI, mice were treated with either dimethyl sulfoxide (control vehicle) or 1, 10, or 30 mg/kg CI-994. Level of acetylated histone H3 expression was increased in the motor cortex and spinal cord of 10 mg/kg CCI-994-treated mice after SCI. CI-994 increased histone H3 acetylation in the myeloperoxidase-positive neutrophils and CD68-positive microglia/macrophages in the spinal cord. Although it did not appear to contribute to corticospinal tract axonal reorganization, intraperitoneal injection of CI-994 promoted behavioral recovery following SCI. Furthermore, administration of CI-994 suppressed neutrophil accumulation, inflammatory cytokine expressions, and neuronal loss as early as 3 days following injury. Thus, our findings indicate that HDAC inhibitors may improve functional recovery following SCI, especially during the early stages of the disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition.Brain Res. 2011 Jun 17;1396:60-8. doi: 10.1016/j.brainres.2011.03.040. Epub 2011 Mar 22. Brain Res. 2011. PMID: 21439269
-
Inhibition of HDAC increases BDNF expression and promotes neuronal rewiring and functional recovery after brain injury.Cell Death Dis. 2020 Aug 18;11(8):655. doi: 10.1038/s41419-020-02897-w. Cell Death Dis. 2020. PMID: 32811822 Free PMC article.
-
Effects of valproic acid, a histone deacetylase inhibitor, on improvement of locomotor function in rat spinal cord injury based on epigenetic science.Iran Biomed J. 2012;16(2):90-100. doi: 10.6091/ibj.1060.2012. Iran Biomed J. 2012. PMID: 22801282 Free PMC article.
-
The Protein Acetylation after Traumatic Spinal Cord Injury: Mechanisms and Therapeutic Opportunities.Int J Med Sci. 2024 Feb 12;21(4):725-731. doi: 10.7150/ijms.92222. eCollection 2024. Int J Med Sci. 2024. PMID: 38464830 Free PMC article. Review.
-
Nanowired drug delivery to enhance neuroprotection in spinal cord injury.CNS Neurol Disord Drug Targets. 2012 Feb;11(1):86-95. doi: 10.2174/187152712799960727. CNS Neurol Disord Drug Targets. 2012. PMID: 22385571 Review.
Cited by
-
Histone Deacetylase Inhibitors: A Novel Strategy in Trauma and Sepsis.Shock. 2019 Sep;52(3):300-306. doi: 10.1097/SHK.0000000000001308. Shock. 2019. PMID: 30601405 Free PMC article. Review.
-
Dose Effects of Histone Deacetylase Inhibitor Tacedinaline (CI-994) on Antipsychotic Haloperidol-Induced Motor and Memory Side Effects in Aged Mice.Front Neurosci. 2021 Oct 6;15:674745. doi: 10.3389/fnins.2021.674745. eCollection 2021. Front Neurosci. 2021. PMID: 34690667 Free PMC article.
-
Histone deacetylase inhibition reduces ventral tegmental area dopamine neuronal hyperexcitability involving AKAP150 signaling following maternal deprivation in juvenile male rats.J Neurosci Res. 2020 Jul;98(7):1457-1467. doi: 10.1002/jnr.24613. Epub 2020 Mar 11. J Neurosci Res. 2020. PMID: 32162391 Free PMC article.
-
Lithium promotes long-term neurological recovery after spinal cord injury in mice by enhancing neuronal survival, gray and white matter remodeling, and long-distance axonal regeneration.Front Cell Neurosci. 2022 Nov 11;16:1012523. doi: 10.3389/fncel.2022.1012523. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36439202 Free PMC article.
-
Epigenetic Regulation Of Axon Regeneration and Glial Activation in Injury Responses.Front Genet. 2019 Jul 9;10:640. doi: 10.3389/fgene.2019.00640. eCollection 2019. Front Genet. 2019. PMID: 31354788 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

