Preservation of rat limbs by hyperbaric carbon monoxide and oxygen
- PMID: 29700404
- PMCID: PMC5919920
- DOI: 10.1038/s41598-018-25070-y
Preservation of rat limbs by hyperbaric carbon monoxide and oxygen
Abstract
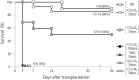
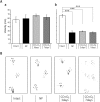
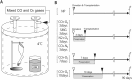
Cold ischemia times ranging from <6 h to as long as 24 h are generally quoted as the limits for attempting the replantation of amputated extremities. In this study, we aimed to assess the effect of hyperbaric carbon monoxide (CO) and oxygen (O2) on rat limb preservation. Donor rat limbs were preserved in a chamber filled with hyperbaric CO and O2 for 3 days (CO + O2 3 days) or 7 days (CO + O2 7 days). Positive and negative control groups were created by using non-preserved limbs (NP) and limbs wrapped in saline-moistened gauze for 3 days (SMG 3 days), respectively. The survival rate of transplanted limbs at postoperative day 90 was 88% in the NP and 86% in the CO + O2 3 days. The corresponding survival rate was 50% in the CO + O2 7 days at postoperative day 90 but was 0% in the SMG 3 days at postoperative day 3. Muscle mass decreased in the CO + O2 3 days and CO + O2 7 days compared with the NP, but sciatic-tibial nerve conduction velocities did not differ. These results indicate that amputated extremities preservation with hyperbaric CO and O2 could extend the time limits of preservation, maintaining their viability for replantation.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Different effects of partial pressure in a high-pressure gaseous mixture of carbon monoxide and oxygen for rat heart preservation.Sci Rep. 2019 May 16;9(1):7480. doi: 10.1038/s41598-019-43905-0. Sci Rep. 2019. PMID: 31097781 Free PMC article.
-
High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation.Int J Mol Sci. 2019 Jun 3;20(11):2719. doi: 10.3390/ijms20112719. Int J Mol Sci. 2019. PMID: 31163581 Free PMC article.
-
Functional evaluation of rat hearts transplanted after preservation in a high-pressure gaseous mixture of carbon monoxide and oxygen.Sci Rep. 2016 Aug 26;6:32120. doi: 10.1038/srep32120. Sci Rep. 2016. PMID: 27562456 Free PMC article.
-
[Gaseous oxygen for protection and conditioning of organs during ischemia].Zentralbl Chir. 1999;124(4):252-9. Zentralbl Chir. 1999. PMID: 10355078 Review. German.
-
[Research progress on preservation of severed limbs].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2000 May;14(3):189-92. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2000. PMID: 12080860 Review. Chinese.
Cited by
-
Different effects of partial pressure in a high-pressure gaseous mixture of carbon monoxide and oxygen for rat heart preservation.Sci Rep. 2019 May 16;9(1):7480. doi: 10.1038/s41598-019-43905-0. Sci Rep. 2019. PMID: 31097781 Free PMC article.
-
High-Pressure Carbon Monoxide and Oxygen Mixture is Effective for Lung Preservation.Int J Mol Sci. 2019 Jun 3;20(11):2719. doi: 10.3390/ijms20112719. Int J Mol Sci. 2019. PMID: 31163581 Free PMC article.
References
-
- Komatsu S, Tamai S. Successful replantation of a completely cut-off thumb. Plast. Reconstr. Surg. 1968;42:375–376. doi: 10.1097/00006534-196810000-00021. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

