Synthesis and conformational analysis of linear homo- and heterooligomers from novel 2-C-branched sugar amino acids (SAAs)
- PMID: 29700416
- PMCID: PMC5919921
- DOI: 10.1038/s41598-018-24927-6
Synthesis and conformational analysis of linear homo- and heterooligomers from novel 2-C-branched sugar amino acids (SAAs)
Abstract
Sugar amino acids (SAAs), as biologically interesting structures bearing both amino and carboxylic acid functional groups represent an important class of multifunctional building blocks. In this study, we develop an easy access to novel SAAs in only three steps starting from nitro compounds in high yields in analytically pure form, easily available by ceric (IV) mediated radical additions. Such novel SAAs have been applied in the assembly of total nine carbopeptoids with the form of linear homo- and heterooligomers for the structural investigations employing circular dichroism (CD) spectroscopy, which suggest that the carbopeptoids emerge a well-extended, left (or right)-handed conformation similar to polyproline II (PPII) helices. NMR studies also clearly demonstrated the presence of ordered secondary structural elements. 2D-ROESY spectra were acquired to identify i+1 NH ↔ i C 1 H, i C 2 H correlations which support the conformational analysis of tetramers by CD spectroscopy. These findings provide interesting information of SAAs and their oligomers as potential scaffolds for discovering new drugs and materials.
Conflict of interest statement
The authors declare no competing interests.
Figures

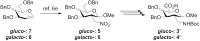





Similar articles
-
Design, synthesis, and NMR structure of linear and cyclic oligomers containing novel furanoid sugar amino acids.Chemistry. 2002 Oct 4;8(19):4365-76. doi: 10.1002/1521-3765(20021004)8:19<4365::AID-CHEM4365>3.0.CO;2-U. Chemistry. 2002. PMID: 12355524
-
Spectroscopic studies of oligomers containing 2,5-trans furanoid sugar amino acids.Chirality. 2008 Sep;20(9):969-72. doi: 10.1002/chir.20538. Chirality. 2008. PMID: 18311778
-
Synthesis and conformational studies of peptidomimetics containing furanoid sugar amino acids and a sugar diacid.J Org Chem. 2000 Oct 6;65(20):6441-57. doi: 10.1021/jo000408e. J Org Chem. 2000. PMID: 11052087
-
The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components.Chem Biodivers. 2004 Aug;1(8):1111-239. doi: 10.1002/cbdv.200490087. Chem Biodivers. 2004. PMID: 17191902 Review.
-
Design and synthesis of chiral alpha,alpha-disubstituted amino acids and conformational study of their oligopeptides.Chem Pharm Bull (Tokyo). 2007 Mar;55(3):349-58. doi: 10.1248/cpb.55.349. Chem Pharm Bull (Tokyo). 2007. PMID: 17329870 Review.
References
-
- Gervay-Hague J, Weathers TM. Pyranosyl Sugar Amino Acids Conjugates: Their BiologicalOrigins, Synthetic Preparations, and Structural Characterization. J. Carbohydr. Chem. 2002;21:867–910. doi: 10.1081/CAR-120016491. - DOI
-
- Chakraborty TK, Jayaprakash S, Ghosh S. Sugar Amino Acid Based Scaffolds - Novel Peptidomimetics and their Potential in CombinatorialSynthesis. Comb. Chem. High. T. Scr. 2002;5:373–387. - PubMed
-
- Schweizer F. Unusual Amino Acids Accessed Through Sugar-Amino Acid Hybrids and Incorporation into Biologically Active Peptides. Trends Glycosci. Glycotechnol. 2003;15:315–328. doi: 10.4052/tigg.15.315. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

