The Correlation Between the Immune and Epithelial-Mesenchymal Transition Signatures Suggests Potential Therapeutic Targets and Prognosis Prediction Approaches in Kidney Cancer
- PMID: 29700419
- PMCID: PMC5919934
- DOI: 10.1038/s41598-018-25002-w
The Correlation Between the Immune and Epithelial-Mesenchymal Transition Signatures Suggests Potential Therapeutic Targets and Prognosis Prediction Approaches in Kidney Cancer
Abstract
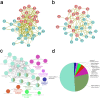
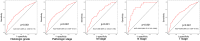
Both epithelial-mesenchymal transition (EMT) and immune regulation are important biological process in malignant tumours. The current research aims to comprehensively explore the potential association between the epithelial-mesenchymal transition (EMT) signature and immune checkpoint signature and its role in predicting the prognosis of clear-cell renal cell carcinoma (ccRCC) patients. EMT-related genes were collected from an experiment-based study and then were investigated using data from the Cancer Genome Atlas. A total of 357 genes were included, and 23 of them that were upregulated and correlated with prognosis were analysed further as core EMT genes in ccRCC. Interestingly, the emerging immune checkpoints CD276, OX40 and TGFB1 were found to be significantly co-expressed with core EMT genes, and TGFB1, CXCR4, IL10, and IL6 were the most important molecules potentially interacting with EMT molecules in our model, as determined from mRNA co-expression and protein-protein interaction network analysis. Additionally, an integrated scoring model based on FOXM1, TIMP1 and IL6 was successfully established to distinguish ccRCC patients with different clinical risks. Our results identified core genes in the EMT-immunophenotyping correlation and evaluated their risk assessment capabilities, providing more potential therapeutic targets and prediction approaches regarding the translational research of treatment and prognosis in ccRCC.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Prognostic value of epithelial-mesenchymal transition markers in clear cell renal cell carcinoma.Aging (Albany NY). 2020 Jan 8;12(1):866-883. doi: 10.18632/aging.102660. Epub 2020 Jan 8. Aging (Albany NY). 2020. PMID: 31915310 Free PMC article.
-
A new thinking: extended application of genomic selection to screen multiomics data for development of novel hypoxia-immune biomarkers and target therapy of clear cell renal cell carcinoma.Brief Bioinform. 2021 Nov 5;22(6):bbab173. doi: 10.1093/bib/bbab173. Brief Bioinform. 2021. PMID: 34237133
-
Epithelial-mesenchymal transition-associated microRNA/mRNA signature is linked to metastasis and prognosis in clear-cell renal cell carcinoma.Sci Rep. 2016 Aug 23;6:31852. doi: 10.1038/srep31852. Sci Rep. 2016. PMID: 27549611 Free PMC article.
-
Epithelial to Mesenchymal Transition in Renal Cell Carcinoma: Implications for Cancer Therapy.Mol Diagn Ther. 2016 Apr;20(2):111-7. doi: 10.1007/s40291-016-0192-5. Mol Diagn Ther. 2016. PMID: 26940073 Review.
-
Is There an Interconnection between Epithelial-Mesenchymal Transition (EMT) and Telomere Shortening in Aging?Int J Mol Sci. 2021 Apr 9;22(8):3888. doi: 10.3390/ijms22083888. Int J Mol Sci. 2021. PMID: 33918710 Free PMC article. Review.
Cited by
-
Development and Clinical Validation of a Seven-Gene Prognostic Signature Based on Multiple Machine Learning Algorithms in Kidney Cancer.Cell Transplant. 2021 Jan-Dec;30:963689720969176. doi: 10.1177/0963689720969176. Cell Transplant. 2021. PMID: 33626918 Free PMC article.
-
TFE3 fusions drive oxidative metabolism and ferroptosis resistance in translocation renal cell carcinoma.EMBO Mol Med. 2025 May;17(5):1041-1070. doi: 10.1038/s44321-025-00221-7. Epub 2025 Mar 27. EMBO Mol Med. 2025. PMID: 40148585 Free PMC article.
-
A novel single-cell based method for breast cancer prognosis.PLoS Comput Biol. 2020 Aug 24;16(8):e1008133. doi: 10.1371/journal.pcbi.1008133. eCollection 2020 Aug. PLoS Comput Biol. 2020. PMID: 32833968 Free PMC article.
-
Expression of FSCN1 and FOXM1 are associated with poor prognosis of adrenocortical carcinoma patients.BMC Cancer. 2019 Nov 29;19(1):1165. doi: 10.1186/s12885-019-6389-3. BMC Cancer. 2019. PMID: 31783819 Free PMC article.
-
Emerging role of tumor cell plasticity in modifying therapeutic response.Signal Transduct Target Ther. 2020 Oct 7;5(1):228. doi: 10.1038/s41392-020-00313-5. Signal Transduct Target Ther. 2020. PMID: 33028808 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

