Exposure to toxic metals triggers unique responses from the rat gut microbiota
- PMID: 29700420
- PMCID: PMC5919903
- DOI: 10.1038/s41598-018-24931-w
Exposure to toxic metals triggers unique responses from the rat gut microbiota
Abstract
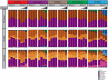
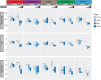
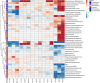
Our understanding of the interaction between the gut microbiota and host health has recently improved dramatically. However, the effects of toxic metal exposure on the gut microbiota remain poorly characterized. As this microbiota creates a critical interface between the external environment and the host's cells, it may play an important role in host outcomes during exposure. We therefore used 16S ribosomal RNA (rRNA) gene sequencing to track changes in the gut microbiota composition of rats exposed to heavy metals. Rats were exposed daily for five days to arsenic, cadmium, cobalt, chromium, nickel, or a vehicle control. Significant changes to microbiota composition were observed in response to high doses of chromium and cobalt, and significant dose-dependent changes were observed in response to arsenic, cadmium and nickel. Many of these perturbations were not uniform across metals. However, bacteria with higher numbers of iron-importing gene orthologs were overly represented after exposure to arsenic and nickel, suggesting some possibility of a shared response. These findings support the utility of the microbiota as a pre-clinical tool for identifying exposures to specific heavy metals. It is also clear that characterizing changes to the functional capabilities of microbiota is critical to understanding responses to metal exposure.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

