Ionic liquids: a brief history
- PMID: 29700779
- PMCID: PMC5988633
- DOI: 10.1007/s12551-018-0419-2
Ionic liquids: a brief history
Abstract
There is no doubt that ionic liquids have become a major subject of study for modern chemistry. We have become used to ever more publications in the field each year, although there is some evidence that this is beginning to plateau at approximately 3500 papers each year. They have been the subject of several major reviews and books, dealing with different applications and aspects of their behaviours. In this article, I will show a little of how interest in ionic liquids grew and developed.
Keywords: Catalysis; Dynamics; Electrochemistry; Green chemistry; Industrial applications; Ionic liquid; Structure; Synthesis.
Conflict of interest statement
Tom Welton declares that he has no conflict of interest.
Figures
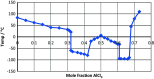


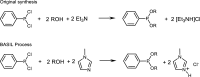

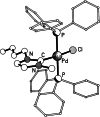
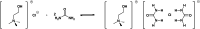
References
-
- Ab Rani MA, Brandt A, Crowhurst L, Dolan A, Lui M, Hassan NH, Hallett JP, Niedermeyer H, Perez-Arlandis JM, Schrems M, To T, Welton T, Wilding R. Understanding the polarity of ionic liquids. Phys Chem Chem Phys. 2011;13:16831–16840. - PubMed
-
- Abai M, Atkins M, Cheun KY, Holbrey JD, Nockemann P, Seddon KR, Srinivasan G, Zou Y (2012) Process for removing metals from hydrocarbons. World Pat., WO2012046057
-
- Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 70–71 - PubMed
-
- Abdul-Sada AK, Avent AG, Parkinton MJ, Ryan TA, Seddon KR, Welton T (1987) The Removal of Oxide Impurities from Room-Temperature Halogenoaluminate Ionic Liquids. J Chem Soc Chem Commun 1643–1644
-
- Abdul-Sada AK, Greenway AM, Seddon KR, Welton T. Upon the existence of [Al3Cl10]− in room-temperature Chloroaluminate ionic liquids. Org Mass Spectrom. 1989;24:917–918.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

