The responses of the inflammatory marker, pentraxin 3, to dietary sodium and potassium interventions
- PMID: 29700922
- PMCID: PMC8031200
- DOI: 10.1111/jch.13273
The responses of the inflammatory marker, pentraxin 3, to dietary sodium and potassium interventions
Abstract
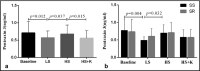
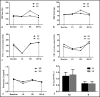
Pentraxin-3 is a sensitive marker of inflammation that plays dual roles, pathogenic and cardioprotective, in the progression of cardiovascular diseases. Inflammation is intimately involved in salt-induced hypertension. We investigated the responses of pentraxin-3 to sodium and potassium supplementation to elucidate the potential role of pentraxin-3 in salt-induced hypertension. A total of 48 participants from northwest China were enrolled. All participants were maintained on a 3-day normal diet, which was sequentially followed by a 7-day low-sodium diet, a 7-day high-sodium diet, and a 7-day high-sodium plus potassium diet. Plasma concentrations of pentraxin-3 were assessed using ELISA. Plasma pentraxin-3 decreased significantly during the low-salt period compared to baseline (0.57 ± 0.19 ng/mL vs 0.72 ± 0.33 ng/mL, P = .012) and increased during the high-salt period (0.68 ± 0.26 ng/mL vs 0.57 ± 0.19 ng/mL, P = .037). Potassium supplementation inhibited salt-induced increase in pentraxin-3 (0.56 ± 0.21 ng/mL vs 0.68 ± 0.26 ng/mL, P = .015). Ln-transformed pentraxin-3 at baseline was inversely correlated with BMI (r = -.349, P = .02), DBP (r = -.414, P = .005), MAP (r = -.360, P = .017). We found a positive correlation between the ln-transformed concentrations of pentraxin-3 and 24-hour urinary sodium during low and high Na+ periods (r = .269, P = .012) and a negative relationship with 24 hours urinary potassium excretion during high-salt and high-salt plus potassium periods (r = -.246, P = .02). These correlations remained significant after adjusting for confounders. Pentraxin-3 responses were more prominent in salt-sensitive individuals than salt-resistant individuals. Dietary salt and potassium interventions significantly altered circulating pentraxin-3.
Keywords: inflammation; pentraxin-3; potassium; salt intake; salt sensitivity.
©2018 Wiley Periodicals, Inc.
Figures


Comment in
-
Relationship between sodium, pentraxin-3 and aldosterone in inflammation and cardiovascular risk.J Clin Hypertens (Greenwich). 2018 May;20(5):932-934. doi: 10.1111/jch.13298. Epub 2018 Apr 27. J Clin Hypertens (Greenwich). 2018. PMID: 29700955 Free PMC article. No abstract available.
Similar articles
-
The Effect of Salt Intake and Potassium Supplementation on Serum Gastrin Levels in Chinese Adults: A Randomized Trial.Nutrients. 2017 Apr 14;9(4):389. doi: 10.3390/nu9040389. Nutrients. 2017. PMID: 28420122 Free PMC article. Clinical Trial.
-
Association of Genetic Variants of Klotho with BP Responses to Dietary Sodium or Potassium Intervention and Long-Term BP Progression.Kidney Blood Press Res. 2022;47(2):94-102. doi: 10.1159/000519839. Epub 2021 Dec 2. Kidney Blood Press Res. 2022. PMID: 34856559
-
Effect of salt intake and potassium supplementation on serum renalase levels in Chinese adults: a randomized trial.Medicine (Baltimore). 2014 Jul;93(6):e44. doi: 10.1097/MD.0000000000000044. Medicine (Baltimore). 2014. PMID: 25058146 Free PMC article. Clinical Trial.
-
Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension.Adv Exp Med Biol. 2017;956:61-84. doi: 10.1007/5584_2016_147. Adv Exp Med Biol. 2017. PMID: 27757935 Review.
-
Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group.Arch Ital Urol Androl. 2015 Jul 7;87(2):105-20. doi: 10.4081/aiua.2015.2.105. Arch Ital Urol Androl. 2015. PMID: 26150027 Review.
Cited by
-
Associations of plasma uromodulin and genetic variants with blood pressure responses to dietary salt interventions.J Clin Hypertens (Greenwich). 2021 Oct;23(10):1897-1906. doi: 10.1111/jch.14347. Epub 2021 Aug 7. J Clin Hypertens (Greenwich). 2021. PMID: 34363725 Free PMC article.
-
Dietary Sodium Intake and Health Indicators: A Systematic Review of Published Literature between January 2015 and December 2019.Adv Nutr. 2020 Sep 1;11(5):1174-1200. doi: 10.1093/advances/nmaa049. Adv Nutr. 2020. PMID: 32449929 Free PMC article.
-
The role of dietary salt in metabolism and energy balance: Insights beyond cardiovascular disease.Diabetes Obes Metab. 2023 May;25(5):1147-1161. doi: 10.1111/dom.14980. Epub 2023 Feb 28. Diabetes Obes Metab. 2023. PMID: 36655379 Free PMC article. Review.
-
Associations of genetic variations in NEDD4L with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults.J Clin Hypertens (Greenwich). 2022 Oct;24(10):1381-1389. doi: 10.1111/jch.14566. Epub 2022 Aug 30. J Clin Hypertens (Greenwich). 2022. PMID: 36039789 Free PMC article.
-
Relationship between sodium, pentraxin-3 and aldosterone in inflammation and cardiovascular risk.J Clin Hypertens (Greenwich). 2018 May;20(5):932-934. doi: 10.1111/jch.13298. Epub 2018 Apr 27. J Clin Hypertens (Greenwich). 2018. PMID: 29700955 Free PMC article. No abstract available.
References
-
- Fang Y, Mu JJ, He LC, Wang SC, Liu ZQ. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt‐sensitive Asians. Hypertension. 2006;48:724‐729. - PubMed
-
- Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt‐sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010;28:527‐535. - PubMed
-
- Zhou MS, Kosaka H, Yoneyama H. Potassium augments vascular relaxation mediated by nitric oxide in the carotid arteries of hypertensive Dahl rats. Am J Hypertens. 2000;13(6 Pt 1):666‐672. - PubMed
-
- Franco V, Oparil S. Salt sensitivity, a determinant of blood pressure, cardiovascular disease and survival. J Am Coll Nutr. 2006;25(3 Suppl):247S‐255S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

