Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson's disease clinical trial
- PMID: 29701258
- PMCID: PMC6175318
- DOI: 10.1002/mds.27376
Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson's disease clinical trial
Abstract
Background: Ubiquitous digital technologies such as smartphone sensors promise to fundamentally change biomedical research and treatment monitoring in neurological diseases such as PD, creating a new domain of digital biomarkers.
Objectives: The present study assessed the feasibility, reliability, and validity of smartphone-based digital biomarkers of PD in a clinical trial setting.
Methods: During a 6-month, phase 1b clinical trial with 44 Parkinson participants, and an independent, 45-day study in 35 age-matched healthy controls, participants completed six daily motor active tests (sustained phonation, rest tremor, postural tremor, finger-tapping, balance, and gait), then carried the smartphone during the day (passive monitoring), enabling assessment of, for example, time spent walking and sit-to-stand transitions by gyroscopic and accelerometer data.
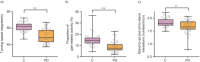
Results: Adherence was acceptable: Patients completed active testing on average 3.5 of 7 times/week. Sensor-based features showed moderate-to-excellent test-retest reliability (average intraclass correlation coefficient = 0.84). All active and passive features significantly differentiated PD from controls with P < 0.005. All active test features except sustained phonation were significantly related to corresponding International Parkinson and Movement Disorder Society-Sponsored UPRDS clinical severity ratings. On passive monitoring, time spent walking had a significant (P = 0.005) relationship with average postural instability and gait disturbance scores. Of note, for all smartphone active and passive features except postural tremor, the monitoring procedure detected abnormalities even in those Parkinson participants scored as having no signs in the corresponding International Parkinson and Movement Disorder Society-Sponsored UPRDS items at the site visit.
Conclusions: These findings demonstrate the feasibility of smartphone-based digital biomarkers and indicate that smartphone-sensor technologies provide reliable, valid, clinically meaningful, and highly sensitive phenotypic data in Parkinson's disease. © 2018 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease; clinical trial; digital biomarkers; digital health; remote patient monitoring.
© 2018 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Figures


References
-
- Sanchez‐Ferro A, Elshehabi M, Godinho C, et al. New methods for the assessment of Parkinson's disease (2005 to 2015): a systematic review. Mov Disord 2016;31:1283‐1292. - PubMed
-
- Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR. Quantitative wearable sensors for objective assessment of Parkinson's disease. Mov Disord 2013;28:1628‐1637. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

