Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: a systematic review and meta-analysis
- PMID: 29701775
- PMCID: PMC6105874
- DOI: 10.1093/jac/dky152
Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: a systematic review and meta-analysis
Abstract
Objectives: The objectives of this study were to explore inter-study heterogeneity in the pharmacokinetics (PK) of orally administered rifampicin, to derive summary estimates of rifampicin PK parameters at standard dosages and to compare these with summary estimates for higher dosages.
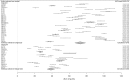
Methods: A systematic search was performed for studies of rifampicin PK published in the English language up to May 2017. Data describing the Cmax and AUC were extracted. Meta-analysis provided summary estimates for PK parameter estimates at standard rifampicin dosages. Heterogeneity was assessed by estimation of the I2 statistic and visual inspection of forest plots. Summary AUC estimates at standard and higher dosages were compared graphically and contextualized using preclinical pharmacodynamic (PD) data.
Results: Substantial heterogeneity in PK parameters was evident and upheld in meta-regression. Treatment duration had a significant impact on the summary estimates for rifampicin PK parameters, with Cmax 8.98 mg/L (SEM 2.19) after a single dose and 5.79 mg/L (SEM 2.14) at steady-state dosing, and AUC 72.56 mg·h/L (SEM 2.60) and 38.73 mg·h/L (SEM 4.33) after single and steady-state dosing, respectively. Rifampicin dosages of at least 25 mg/kg are required to achieve plasma PK/PD targets defined in preclinical studies.
Conclusions: Vast inter-study heterogeneity exists in rifampicin PK parameter estimates. This is not explained by the available modifying variables. The recommended dosage of rifampicin should be increased to improve efficacy. This study provides an important point of reference for understanding rifampicin PK at standard dosages as efforts to explore higher dosing strategies continue in this field.
Figures


References
-
- Zumla A, Nahid P, Cole ST.. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov 2013; 12: 388–404. - PubMed
-
- WHO, ‘StopTB’ Initiative. Guidelines for Treatment of Tuberculosis, Fourth Edition http://www.who.int/tb/publications/2010/9789241547833/en/.
-
- van Ingen J, Aarnoutse RE, Donald PR. et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 2011; 52: e194–9. - PubMed
-
- Peloquin C. Therapeutic drug monitoring: principles and applications in mycobacterial infections. Drug Therapy 1992; 22: 31–6.
-
- Peloquin CA, Nitta AT, Burman WJ. et al. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother 1996; 30: 919–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

