Clearance of Somatic Mutations at Remission and the Risk of Relapse in Acute Myeloid Leukemia
- PMID: 29702001
- PMCID: PMC6008108
- DOI: 10.1200/JCO.2017.77.6757
Clearance of Somatic Mutations at Remission and the Risk of Relapse in Acute Myeloid Leukemia
Abstract
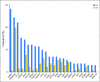
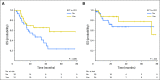
Purpose The aim of the current study was to determine whether the degree of mutation clearance at remission predicts the risk of relapse in patients with acute myeloid leukemia (AML). Patients and Methods One hundred thirty-one previously untreated patients with AML who received intensive induction chemotherapy and attained morphologic complete remission (CR) at day 30 were studied. Pretreatment and CR bone marrow were analyzed using targeted capture DNA sequencing. We analyzed the association between mutation clearance (MC) on the basis of variant allele frequency (VAF) at CR (MC2.5: if the VAF of residual mutations was < 2.5%; MC1.0: if the VAF was < 1%; and complete MC [CMC]: if no detectable residual mutations) and event-free survival, overall survival (OS), and cumulative incidence of relapse (CIR). Results MC1.0 and CMC were associated with significantly better OS (2-year OS: 75% v 61% in MC1.0 v non-MC1.0; P = .0465; 2-year OS: 77% v 60% in CMC v non-CMC; P = .0303) and lower CIR (2-year CIR: 26% v 46% in MC1.0 v non-MC 1.0; P = .0349; 2 year-CIR: 24% v 46% in CMC v non-CMC; P = .03), whereas there was no significant difference in any of the above outcomes by MC2.5. Multivariable analysis adjusting for age, cytogenetic risk, allogeneic stem-cell transplantation, and flow cytometry-based minimal residual disease revealed that patients with CMC had significantly better event-free survival (hazard ratio [HR], 0.43; P = .0083), OS (HR, 0.47; P = .04), and CIR (HR, 0.27; P < .001) than did patients without CMC. These prognostic associations were stronger when preleukemic mutations, such as DNMT3A, TET2, and ASXL1, were removed from the analysis. Conclusion Clearance of somatic mutation at CR, particularly in nonpreleukemic genes, was associated with significantly better survival and less risk of relapse. Somatic mutations in nonpreleukemic genes may function as a molecular minimal residual disease marker in AML.
Trial registration: ClinicalTrials.gov NCT01025154 NCT01289457 NCT02115295.
Figures


References
-
- Rücker FG, Schlenk RF, Bullinger L, et al. : TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 119:2114-2121, 2012 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

