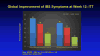
Improvement in Gastrointestinal Symptoms After Cognitive Behavior Therapy for Refractory Irritable Bowel Syndrome
- PMID: 29702118
- PMCID: PMC6035059
- DOI: 10.1053/j.gastro.2018.03.063
Improvement in Gastrointestinal Symptoms After Cognitive Behavior Therapy for Refractory Irritable Bowel Syndrome
Erratum in
-
Correction.Gastroenterology. 2018 Oct;155(4):1281. doi: 10.1053/j.gastro.2018.09.049. Epub 2018 Sep 27. Gastroenterology. 2018. PMID: 30268377 No abstract available.
Abstract
Background & aims: There is an urgent need for safe treatments for irritable bowel syndrome (IBS) that relieve treatment-refractory symptoms and their societal and economic burden. Cognitive behavior therapy (CBT) is an effective treatment that has not been broadly adopted into routine clinical practice. We performed a randomized controlled trial to assess clinical responses to home-based CBT compared with clinic-based CBT and patient education.
Methods: We performed a prospective study of 436 patients with IBS, based on Rome III criteria, at 2 tertiary centers from August 23, 2010, through October 21, 2016. Subjects (41.4 ± 14.8 years old; 80% women) were randomly assigned to groups that received the following: standard-CBT (S-CBT, n = 146, comprising 10 weekly, 60-minute sessions that emphasized the provision of information about brain-gut interactions; self-monitoring of symptoms, their triggers, and consequences; muscle relaxation; worry control; flexible problem solving; and relapse prevention training), or 4 sessions of primarily home-based CBT requiring minimal therapist contact (MC-CBT, n = 145), in which patients received home-study materials covering the same procedures as S-CBT), or 4 sessions of IBS education (EDU, n = 145) that provided support and information about IBS and the role of lifestyle factors such as stress, diet, and exercise. The primary outcome was global improvement of IBS symptoms, based on the IBS-version of the Clinical Global Impressions-Improvement Scale. Ratings were performed by patients and board-certified gastroenterologists blinded to treatment allocation. Efficacy data were collected 2 weeks, 3 months, and 6 months after treatment completion.
Results: A higher proportion of patients receiving MC-CBT reported moderate to substantial improvement in gastrointestinal symptoms 2 weeks after treatment (61.0% based on ratings by patients and 55.7% based on ratings by gastroenterologists) than those receiving EDU (43.5% based on ratings patients and 40.4% based on ratings by gastroenterologists) (P < .05). Gastrointestinal symptom improvement, rated by gastroenterologists, 6 months after the end of treatment also differed significantly between the MC-CBT (58.4%) and EDU groups (44.8%) (P = .05). Formal equivalence testing applied across multiple contrasts indicated that MC-CBT is at least as effective as S-CBT in improving IBS symptoms. Patients tended to be more satisfied with CBT vs EDU (P < .05) based on immediate posttreatment responses to the Client Satisfaction Questionnaire. Symptom improvement was not significantly related to concomitant use of medications.
Conclusions: In a randomized controlled trial, we found that a primarily home-based version of CBT produced significant and sustained gastrointestinal symptom improvement for patients with IBS compared with education. Clinicaltrials.gov no.: NCT00738920.
Keywords: Brain-Gut Interactions; Disease Management; Functional Gastrointestinal Disorder; Value-Based Health Care.
Copyright © 2018 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Intending to Treat Patients With Irritable Bowel Syndrome With Cognitive-Behavioral Therapy.Gastroenterology. 2018 Dec;155(6):2024. doi: 10.1053/j.gastro.2018.05.054. Epub 2018 Sep 18. Gastroenterology. 2018. PMID: 30240662 No abstract available.
-
Reply.Gastroenterology. 2018 Dec;155(6):2024-2025. doi: 10.1053/j.gastro.2018.11.013. Epub 2018 Nov 9. Gastroenterology. 2018. PMID: 30419207 No abstract available.
Similar articles
-
Specific and common mediators of gastrointestinal symptom improvement in patients undergoing education/support vs. cognitive behavioral therapy for irritable bowel syndrome.J Consult Clin Psychol. 2021 May;89(5):435-453. doi: 10.1037/ccp0000648. J Consult Clin Psychol. 2021. PMID: 34124927 Free PMC article.
-
Durability and Decay of Treatment Benefit of Cognitive Behavioral Therapy for Irritable Bowel Syndrome: 12-Month Follow-Up.Am J Gastroenterol. 2019 Feb;114(2):330-338. doi: 10.1038/s41395-018-0396-x. Am J Gastroenterol. 2019. PMID: 30429592 Free PMC article. Clinical Trial.
-
Factors Associated With Efficacy of Cognitive Behavior Therapy vs Education for Patients With Irritable Bowel Syndrome.Clin Gastroenterol Hepatol. 2019 Jul;17(8):1500-1508.e3. doi: 10.1016/j.cgh.2018.10.033. Epub 2018 Oct 26. Clin Gastroenterol Hepatol. 2019. PMID: 30613000 Free PMC article. Clinical Trial.
-
Cognitive behaviour therapy for irritable bowel syndrome.Eur J Gastroenterol Hepatol. 2005 Jan;17(1):11-4. doi: 10.1097/00042737-200501000-00003. Eur J Gastroenterol Hepatol. 2005. PMID: 15647633 Review.
-
Predictors of outcome in cognitive and behavioural interventions for irritable bowel syndrome. A meta-analysis.J Gastrointestin Liver Dis. 2018 Sep;27(3):257-263. doi: 10.15403/jgld.2014.1121.273.bab. J Gastrointestin Liver Dis. 2018. PMID: 30240469 Review.
Cited by
-
Pharmacological and Non-pharmacological Treatments of Irritable Bowel Syndrome and Their Impact on the Quality of Life: A Literature Review.Cureus. 2020 Jul 21;12(7):e9324. doi: 10.7759/cureus.9324. Cureus. 2020. PMID: 32850202 Free PMC article. Review.
-
The Role of Virtual Reality in the Management of Irritable Bowel Syndrome.Curr Gastroenterol Rep. 2024 Nov;26(11):294-303. doi: 10.1007/s11894-024-00940-w. Epub 2024 Aug 13. Curr Gastroenterol Rep. 2024. PMID: 39136889 Free PMC article. Review.
-
Irritable Bowel Syndrome in the Elderly Population: A Comprehensive Review.Cureus. 2024 Aug 29;16(8):e68156. doi: 10.7759/cureus.68156. eCollection 2024 Aug. Cureus. 2024. PMID: 39347183 Free PMC article. Review.
-
Gut liver brain axis in diseases: the implications for therapeutic interventions.Signal Transduct Target Ther. 2023 Dec 6;8(1):443. doi: 10.1038/s41392-023-01673-4. Signal Transduct Target Ther. 2023. PMID: 38057297 Free PMC article. Review.
-
Irritable Bowel Syndrome: Treating the Gut and Brain/Mind at the Same Time.Cureus. 2023 Aug 13;15(8):e43404. doi: 10.7759/cureus.43404. eCollection 2023 Aug. Cureus. 2023. PMID: 37706135 Free PMC article. Review.
References
-
- Hungin APS, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharm Ther. 2005;21(11):1365–1375. - PubMed
-
- Camilleri M, Choi MG. Review article: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11(1):3–15. - PubMed
-
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–754. - PubMed
-
- Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376(26):2566–2578. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous