Quinolinic acid and glutamatergic neurodegeneration in Caenorhabditis elegans
- PMID: 29702159
- PMCID: PMC6054819
- DOI: 10.1016/j.neuro.2018.04.015
Quinolinic acid and glutamatergic neurodegeneration in Caenorhabditis elegans
Abstract
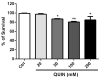
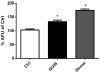
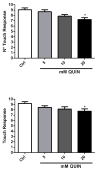
Quinolinic acid (QUIN) is an endogenous neurotoxin that acts as an N-methyl-D-aspartate receptor (NMDAR) agonist generating a toxic cascade, which can lead to neurodegeneration. The action of QUIN in Caenorhabditis elegans and the neurotoxins that allow the study of glutamatergic system disorders have not been carefully addressed. The effects of QUIN on toxicological and behavioral parameters in VM487 and VC2623 transgenic, as well as wild-type (WT) animals were performed to evaluate whether QUIN could be used as a neurotoxin in C. elegans. QUIN reduced survival of WT worms in a dose-dependent manner. A sublethal dose of QUIN (20 mM) increased reactive oxygen species (ROS) levels in an nmr-1/NMDAR-dependent manner, activated the DAF-16/FOXO transcription factor, and increased expression of the antioxidant enzymes, superoxide dismutase-3, glutathione S-transferase-4, and heat shock protein-16.2. QUIN did not change motor behavioral parameters, but altered the sensory behavior in N2 and VM487 worms. Notably, the effect of QUIN on the sensory behavioral parameters might occur, at least in part, secondary to increased ROS. However, the touch response behavior indicates a mechanism of action that is independent of ROS generation. In addition, non-lethal doses of QUIN triggered neurodegeneration in glutamatergic neurons. Our findings indicate that C. elegans might be useful as a model for studies of QUIN as a glutamatergic neurotoxin in rodent models.
Keywords: C. elegans; Glutamatergic system; NMDA; Neurodegeneration; Quinolinic acid.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
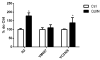







References
-
- Chen Y, Meininger V, Guillemin GJ. Recent advances in the treatment of amyotrophic lateral sclerosis. Emphasis on kynurenine pathway inhibitors. Cent Nerv Syst Agents Med Chem. 2009;9(1):32–9. - PubMed
-
- Braidy N, et al. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res. 2009;16(1):77–86. - PubMed
-
- Perez-De La Cruz V, Konigsberg M, Santamaria A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets. 2007;6(6):398–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

