MGST1, a GSH transferase/peroxidase essential for development and hematopoietic stem cell differentiation
- PMID: 29702404
- PMCID: PMC6006721
- DOI: 10.1016/j.redox.2018.04.013
MGST1, a GSH transferase/peroxidase essential for development and hematopoietic stem cell differentiation
Abstract
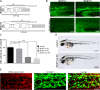
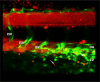
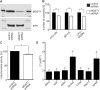
We show for the first time that, in contrast to other glutathione transferases and peroxidases, deletion of microsomal glutathione transferase 1 (MGST1) in mice is embryonic lethal. To elucidate why, we used zebrafish development as a model system and found that knockdown of MGST1 produced impaired hematopoiesis. We show that MGST1 is expressed early during zebrafish development and plays an important role in hematopoiesis. High expression of MGST1 was detected in regions of active hematopoiesis and co-expressed with markers for hematopoietic stem cells. Further, morpholino-mediated knock-down of MGST1 led to a significant reduction of differentiated hematopoietic cells both from the myeloid and the lymphoid lineages. In fact, hemoglobin was virtually absent in the knock-down fish as revealed by diaminofluorene staining. The impact of MGST1 on hematopoiesis was also shown in hematopoietic stem/progenitor cells (HSPC) isolated from mice, where it was expressed at high levels. Upon promoting HSPC differentiation, lentiviral shRNA MGST1 knockdown significantly reduced differentiated, dedicated cells of the hematopoietic system. Further, MGST1 knockdown resulted in a significant lowering of mitochondrial metabolism and an induction of glycolytic enzymes, energetic states closely coupled to HSPC dynamics. Thus, the non-selenium, glutathione dependent redox regulatory enzyme MGST1 is crucial for embryonic development and for hematopoiesis in vertebrates.
Keywords: Embryonic development; Hematopoiesis; Microsomal glutathione transferase/peroxidase; Redox regulation.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. - PubMed
-
- Rinaldi R., Eliasson E., Swedmark S., Morgenstern R. Reactive intermediates and the dynamics of glutathione transferases. Drug Metab. Dispos. 2002;30(10):1053–1058. - PubMed
-
- Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. - PubMed
-
- Arner E.S. Focus on mammalian thioredoxin reductases–important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790(6):495–526. - PubMed
-
- Brigelius-Flohe R., Maiorino M. Glutathione peroxidases. Biochim. Biophys. Acta. 2013;1830(5):3289–3303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

