Synthesis and Pharmacological Studies of Unprecedented Fused Pyridazino[3',4':5,6][1,2,4]triazino[3,4- b][1,3,4]thiadiazine Derivatives
- PMID: 29702549
- PMCID: PMC6099593
- DOI: 10.3390/molecules23051024
Synthesis and Pharmacological Studies of Unprecedented Fused Pyridazino[3',4':5,6][1,2,4]triazino[3,4- b][1,3,4]thiadiazine Derivatives
Abstract
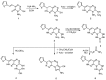
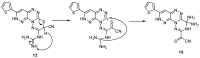
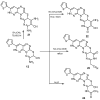
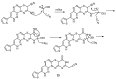
A novel fused system with three or four fused rings—pyridazino[3′,4′:5,6][1,2,4]triazino[4,3-b][1,2,4,5]tetrazine and pyridazino[3′,4′:5,6][1,2,4]triazino[3,4-b]pyrimido[4,5-e][1,3,4]thiadiazine was obtained from the starting materials 4(6H)-amino-3-hydrazino-7-(2-thienyl)pyridazino[3,4-e][1,2,4]-triazine 2 and 9-amino-3-(2-thienyl)-2H,8H-pyridazino[3′,4′:5,6][1,2,4]triazino[3,4-b][1,3,4]thiadiazine-8-carbonitrile 12. Each of the starting compounds was subjected to a number of cyclization reactions to obtain a series of new heterocyclic fused systems, 3⁻10 and 13⁻23, via bifunctional reagents. Some of the synthesized compounds were screened against three cell lines including HepG2, HCT-116 and MCF-7 to discover their anticancer activity. The synthesized compounds were characterized depending on their elemental analyses and spectral data.
Keywords: antitumor activity; biological applications; cyclization reactions; pyridazino[3′,4′:5,6][1,2,4]triazino[4,3-b][1,2,4,5]tetrazine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Patel A., Mehta G.A. Synthesis and characterization of some pyrimidine-quinoline clubbed molecules and their microbicidal efficacy. J. Saudi Chem. Soc. 2010;14:203–208. doi: 10.1016/j.jscs.2010.02.012. - DOI
-
- Gouda M.A., Eldien H.F., Girges M.M., Berghot M.A. Synthesis and antitumor evaluation of thiophene-based azo dyes incorporating pyrazolone moiety. J. Saudi Chem. Soc. 2016;20:151–157. doi: 10.1016/j.jscs.2012.06.004. - DOI
-
- Desai N.C., Dodiya A.M., Rajpara K.M., Rupala Y.M. Synthesis and antimicrobial screening of 1,3,4-oxadiazole and clubbed thiophene derivatives. J. Saudi Chem. Soc. 2014;18:255–261. doi: 10.1016/j.jscs.2011.06.020. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

