CDG Therapies: From Bench to Bedside
- PMID: 29702557
- PMCID: PMC5983582
- DOI: 10.3390/ijms19051304
CDG Therapies: From Bench to Bedside
Abstract
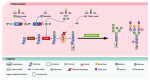
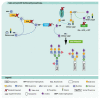
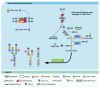
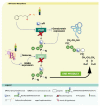
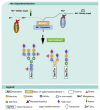
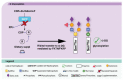
Congenital disorders of glycosylation (CDG) are a group of genetic disorders that affect protein and lipid glycosylation and glycosylphosphatidylinositol synthesis. More than 100 different disorders have been reported and the number is rapidly increasing. Since glycosylation is an essential post-translational process, patients present a large range of symptoms and variable phenotypes, from very mild to extremely severe. Only for few CDG, potentially curative therapies are being used, including dietary supplementation (e.g., galactose for PGM1-CDG, fucose for SLC35C1-CDG, Mn2+ for TMEM165-CDG or mannose for MPI-CDG) and organ transplantation (e.g., liver for MPI-CDG and heart for DOLK-CDG). However, for the majority of patients, only symptomatic and preventive treatments are in use. This constitutes a burden for patients, care-givers and ultimately the healthcare system. Innovative diagnostic approaches, in vitro and in vivo models and novel biomarkers have been developed that can lead to novel therapeutic avenues aiming to ameliorate the patients’ symptoms and lives. This review summarizes the advances in therapeutic approaches for CDG.
Keywords: animal models; biomarkers; clinical trials; congenital disorders of glycosylation (CDG); diagnosis; dietary supplementation; galactose; mannose; pharmacological chaperones; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Marques-da-Silva D., dos Reis Ferreira V., Monticelli M., Janeiro P., Videira P.A., Witters P., Jaeken J., Cassiman D. Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J. Inherit. Metab. Dis. 2017;40:195–207. doi: 10.1007/s10545-016-0012-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

