Plasma rich in growth factors membrane as coadjuvant treatment in the surgery of ocular surface disorders
- PMID: 29702971
- PMCID: PMC5944476
- DOI: 10.1097/MD.0000000000010242
Plasma rich in growth factors membrane as coadjuvant treatment in the surgery of ocular surface disorders
Abstract
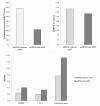
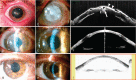
To evaluate the safety and efficacy of the surgical use of plasma rich in growth factors fibrin membrane (mPRGF) in different ocular surface pathologies.Fifteen patients with different corneal and conjunctival diseases were included in the study. Patients were grouped according to the use of mPRGF as graft (corneal and/or conjunctival) or dressing; they were also grouped according to the surgical subgroup of intervention (persistent corneal ulcer [PCU], keratoplasty, superficial keratectomy, corneal perforation, and pterygium). Best corrected visual acuity, intraocular pressure (IOP), inflammation control time (ICT), mPRGF AT (PRGF membrane absorption time), and the healing time of the epithelial defect (HTED) were evaluated throughout the clinical follow-up time. Safety assessment was also performed reporting all adverse events.mPRGF showed a total closure of the defect in 13 of 15 patients (86.7%) and a partial closure in 2 patients (13.3%). The mean follow-up time was 11.1 ± 4.2 (4.8-22.8) months, the mean ICT was 2.5 ± 1.1 (1.0-4.0) months, the mean mPRGF AT was 12.4 ± 2.0 (10.0-16.0) days, and for the global HTED the mean was 2.9 ± 1.2 (1-4.8) months. Results showed an improvement in BCVA in all patients, with an overall improvement of 2.9 in Vision Lines. The BCVA significantly improved (P < .05) in the groups of corneal graft and dressing. In the PCU subgroup (6 patients), the healing time of epithelial defect was significantly reduced (P < .05) in patients treated only with the mPRGF in comparison to those which mPRGF therapy was associated to the amniotic membrane. The IOP remained stable (P > .05) throughout the clinical follow-up time. No adverse events were reported after mPRGF use.The mPRGF is effective and safe as coadjuvant treatment in surgeries related with ocular surface disorders, being an alternative to the use of amniotic membrane. The mPRGF accelerates tissue regeneration after ocular surface surgery thus minimizing inflammation and fibrosis.
Conflict of interest statement
The authors declare the following competing financial interest(s): Eduardo Anitua is the Scientific Director of BTI Biotechnology Institute, Gorka Orive and Francisco Muruzabal are scientists at BTI Biotechnology Institute, a dental implant company, who investigates in the fields of oral implantology and PRGF-Endoret technology. The rest of the authors declare that there is no conflict of interest regarding the publication of this paper.
Figures




Similar articles
-
Use of autologous plasma rich in growth factors fibrin membrane in the surgical management of ocular surface diseases.Int Ophthalmol. 2021 Jul;41(7):2347-2358. doi: 10.1007/s10792-021-01788-z. Epub 2021 Mar 21. Int Ophthalmol. 2021. PMID: 33745034
-
[Amniotic membrane graft in ocular surface disease. Prospective study with 31 cases].J Fr Ophtalmol. 2001 Oct;24(8):798-812. J Fr Ophtalmol. 2001. PMID: 11894530 French.
-
Development and optimization of a personalized fibrin membrane derived from the plasma rich in growth factors technology.Exp Eye Res. 2021 Feb;203:108402. doi: 10.1016/j.exer.2020.108402. Epub 2020 Dec 14. Exp Eye Res. 2021. PMID: 33326809
-
Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review.Cell Tissue Bank. 2017 Jun;18(2):193-204. doi: 10.1007/s10561-017-9618-5. Epub 2017 Mar 2. Cell Tissue Bank. 2017. PMID: 28255771 Review.
-
Eye platelet-rich plasma in the treatment of ocular surface disorders.Curr Opin Ophthalmol. 2015 Jul;26(4):325-32. doi: 10.1097/ICU.0000000000000169. Curr Opin Ophthalmol. 2015. PMID: 26058033 Review.
Cited by
-
Fibrin-Plasma Rich in Growth Factors Membrane for the Treatment of a Rabbit Alkali-Burn Lesion.Int J Mol Sci. 2021 May 25;22(11):5564. doi: 10.3390/ijms22115564. Int J Mol Sci. 2021. PMID: 34070266 Free PMC article.
-
Plasma Rich in Growth Factors as an Adjuvant Agent in Non-Penetrating Deep Sclerectomy.J Clin Med. 2023 May 22;12(10):3604. doi: 10.3390/jcm12103604. J Clin Med. 2023. PMID: 37240710 Free PMC article.
-
Membrane of Plasma Rich in Growth Factors in Primary Pterygium Surgery Compared to Amniotic Membrane Transplantation and Conjunctival Autograft.J Clin Med. 2021 Dec 6;10(23):5711. doi: 10.3390/jcm10235711. J Clin Med. 2021. PMID: 34884413 Free PMC article.
-
Real-World Outcomes of Allogeneic Immunosafe Plasma Rich in Growth Factors Eye Drops for Refractory Ocular Surface Diseases: A Prospective Observational Study.Ophthalmol Ther. 2025 Jul 25. doi: 10.1007/s40123-025-01211-1. Online ahead of print. Ophthalmol Ther. 2025. PMID: 40715787
-
Growth Factors in Oral Tissue Engineering: New Perspectives and Current Therapeutic Options.Biomed Res Int. 2021 Jan 6;2021:8840598. doi: 10.1155/2021/8840598. eCollection 2021. Biomed Res Int. 2021. PMID: 33506039 Free PMC article.
References
-
- Feng Y, Borrelli M, Reichl S, et al. Review of alternative carrier materials for ocular surface reconstruction. Curr Eye Res 2014;39:541–52. - PubMed
-
- Alemañy González J, Camacho Ruaigip F. Usos de la membrana amniótica humana en oftalmología. Rev Cuba Oftalmol 2006;19:1–7.
-
- Fernandes M, Sridhar M, Sangwan V, et al. Amniotic membrane transplantation for ocular surface reconstruction. Cornea 2005;24:643–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

