Bortezomib enhances radiosensitivity in oral cancer through inducing autophagy-mediated TRAF6 oncoprotein degradation
- PMID: 29703234
- PMCID: PMC5921410
- DOI: 10.1186/s13046-018-0760-0
Bortezomib enhances radiosensitivity in oral cancer through inducing autophagy-mediated TRAF6 oncoprotein degradation
Abstract
Background: Oral squamous cell carcinoma (OSCC) is a malignant tumor that may occur anywhere within the oral cavity. The survival rate of OSCC patients has not improved over the past decades due to its heterogeneous etiology, genetic aberrations, and treatment outcomes. We investigated the role of tumor necrosis factor receptor-associated factor 6 (TRAF6) in OSCC cells treated with bortezomib (a proteasome inhibitor) combined with irradiation (IR) treatment.
Methods: The effects of combined treatment in OSCC cells were investigated using assays of cell viability, autophagy, apoptosis, western blotting, and immunofluorescence staining. The ubiquitination of proteins was analyzed by immunoprecipitation. Stable knockdown of TRAF6 in OSCC cells was constructed with lentivirus. The xenograft murine models were used to observe tumor growth.
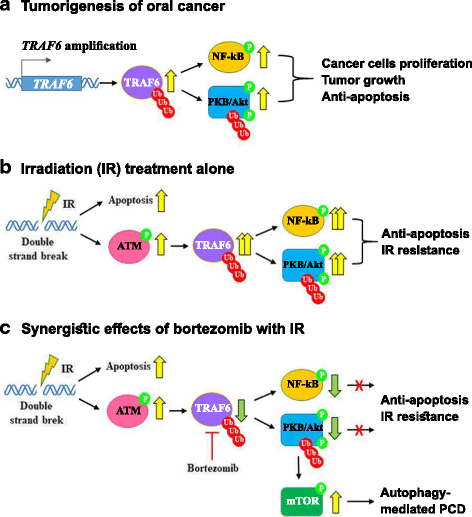
Results: We found synergistic effects of bortezomib and IR on the viability of human oral cancer cells. The combination of bortezomib and IR treatment induced autophagic cell death. Furthermore, bortezomib inhibited IR-induced TRAF6 ubiquitination and inhibited TRAF6-mediated Akt activation. Bortezomib reduced TRAF6 protein expression through autophagy-mediated lysosomal degradation. TRAF6 played an oncogenic role in tumorigenesis of human oral cancer cells and oral tumor growth was suppressed by bortezomib and IR treatment. In addition, OSCC patients with expression of TRAF6 showed a trend towards poorer cancer-specific survival when compared with patients without TRAF6 expression.
Conclusions: A combination of a proteasome inhibitor, IR treatment and TRAF6 inhibition could be a novel therapeutic strategy in OSCC.
Keywords: Autophagy; Oral squamous cell carcinoma; Radiation; TRAF6; Ubiquitination.
Conflict of interest statement
Ethics approval and consent to participate
Clinical tissues were collected from patients who received curative surgery for oral squamous cell carcinoma at National Cheng Kung University Hospital, Taiwan. All experiments on mice were performed according to the guidelines of our institute (the Guide for Care and Use of Laboratory Animals, National Cheng Kung University). The animal use protocol listed below has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) (Approval No: 99138).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Montagnani F, Fornaro L, Frumento P, Vivaldi C, Falcone A, Fioretto L. Multimodality treatment of locally advanced squamous cell carcinoma of the oesophagus: a comprehensive review and network meta-analysis. Crit Rev Oncol Hematol. 2017;114:24–32. doi: 10.1016/j.critrevonc.2017.03.024. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

