Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury
- PMID: 29703241
- PMCID: PMC5923011
- DOI: 10.1186/s12974-018-1160-2
Human immunodeficiency virus type 1 (HIV-1)-mediated neuroinflammation dysregulates neurogranin and induces synaptodendritic injury
Abstract
Background: Human immunodeficiency virus type 1 (HIV-1)-associated neurocognitive disorder (HAND) is a common outcome of a majority of HIV-1-infected subjects and is associated with synaptodendritic damage. Neurogranin (Ng), a postsynaptic protein, and calmodulin (CaM) are two important players of synaptic integrity/functions. The biological role of Ng in the context of HAND is unknown.
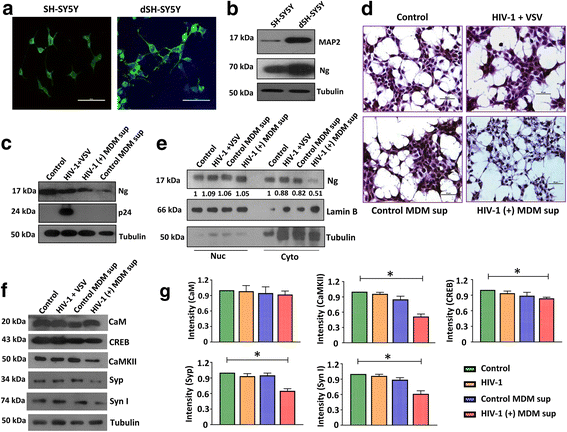
Methods: We compared the expression of Ng in frontal cortex (FC) tissues from control and HIV-1-positive subjects with and without HAND by immunohistochemistry, western blot, and qRT-PCR. The interaction between Ng and CaM was analyzed by co-immunoprecipitation. Ng, microtubule-associated protein 2 (MAP2), CaM, CaM-dependent protein kinase II (CaMKII), CREB, synaptophysin (Syp), and synapsin I (Syn I) expressions were evaluated by western blot using FC tissue lysates and differentiated SH-SY5Y (dSH-SY5Y) cells. Identification of inflammatory factors related to Ng loss was accomplished by exposing dSH-SY5Y cells to HIV-1 and mock-infected monocyte-derived macrophage (MDM) supernatants or HIV-1 NLYU2 pseudotyped with VSV-G-Env. Levels of interleukin (IL)-1β, IL-8, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, MCP-2, and CXCL5 in MDM supernatants were measured by ELISA. Association of IL-1β and IL-8 to Ng expression in context of HIV-1 infection was evaluated in the presence or absence of neutralizing antibodies against these cytokines.
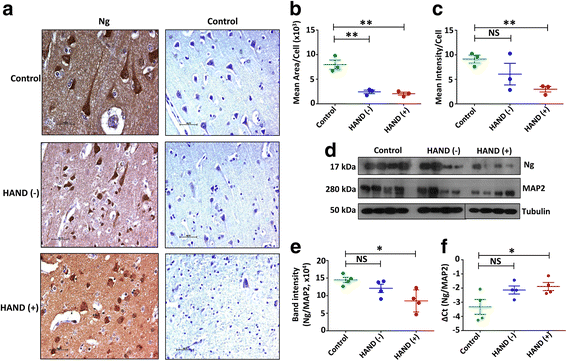
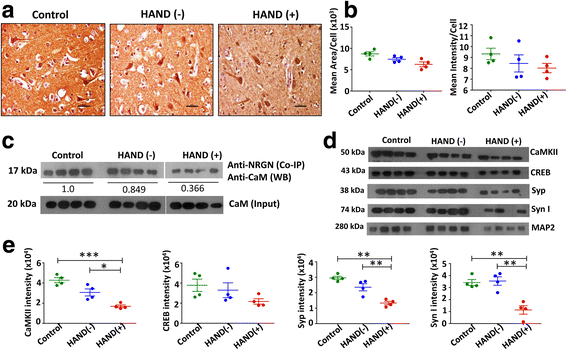
Results: Expression level of Ng was reduced significantly in FC of HAND-positive (HAND+) patients compared to uninfected individuals. Although no difference was found in CaM expression, interaction between Ng and CaM was reduced in HAND+ patients, which was associated with decreased level of CaMKII, a downstream signaling molecule of CaM pathway. This in turn resulted in reduction of synaptic markers, Syp and Syn I. HIV-1 infection directly had no considerable effect on dysregulation of Ng expression in dSH-SY5Y cells, whereas high amount of pro-inflammatory IL-1β and IL-8 in HIV-1-infected MDM supernatants was associated with significant reduction in Ng expression.
Conclusions: Synaptic damage in HAND+ patients could be a result of abrogation of Ng through HIV-1-induced inflammation that dysregulates Ng-CaM interaction and downstream signaling cascades associated with synaptodendritic functions. This is the first study evaluating the potential role of Ng in the context of HIV-1 neuropathogenesis.
Keywords: Calmodulin; Frontal cortex; HIV-1; Neurogranin; Synaptodendritic damage.
Conflict of interest statement
Ethics approval and consent to participate
FC tissues and formalin-fixed paraffin-sectioned slides were obtained from NNTC and CAMACS using appropriate institutional IRB and CORID approval.
Consent for publication
Agree to publish as required by the funding agency (NIH). The subjects enrolled in MACS and NNTC consent to publish results utilizing the samples/specimen provided by them for the research study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. - DOI - PMC - PubMed
-
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. - DOI - PMC - PubMed
-
- Krucker T, Siggins GR, McNamara RK, Lindsley KA, Dao A, Allison DW, De Lecea L, Lovenberg TW, Sutcliffe JG, Gerendasy DD. Targeted disruption of RC3 reveals a calmodulin-based mechanism for regulating metaplasticity in the hippocampus. J Neurosci. 2002;22:5525–5535. doi: 10.1523/JNEUROSCI.22-13-05525.2002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

