Zika virus-induced hyper excitation precedes death of mouse primary neuron
- PMID: 29703263
- PMCID: PMC5922018
- DOI: 10.1186/s12985-018-0989-4
Zika virus-induced hyper excitation precedes death of mouse primary neuron
Abstract
Background: Zika virus infection in new born is linked to congenital syndromes, especially microcephaly. Studies have shown that these neuropathies are the result of significant death of neuronal progenitor cells in the central nervous system of the embryo, targeted by the virus. Although cell death via apoptosis is well acknowledged, little is known about possible pathogenic cellular mechanisms triggering cell death in neurons.
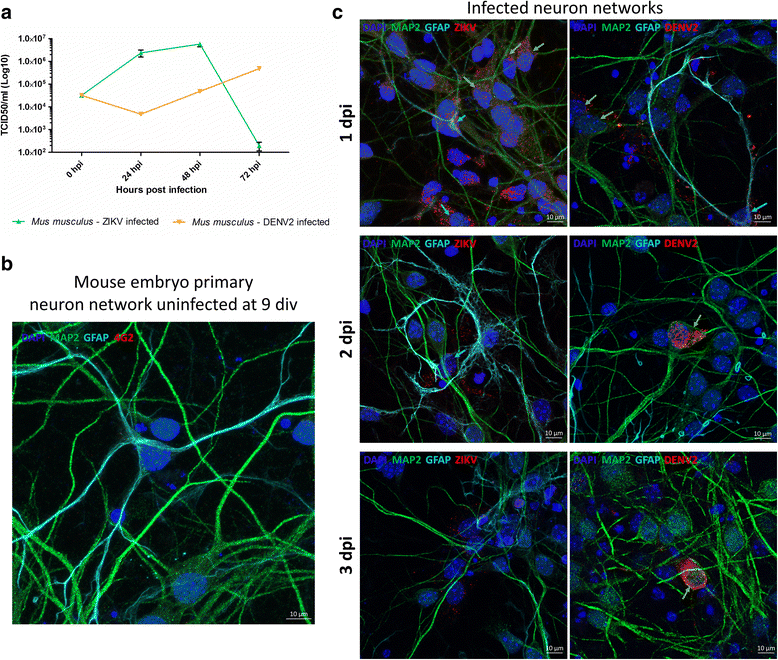
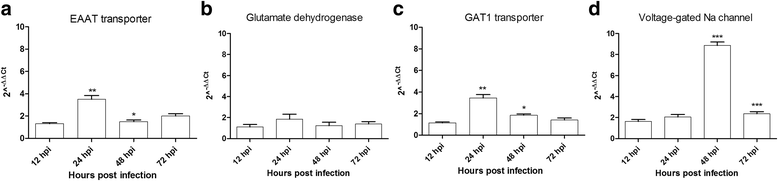
Methods: We used in vitro embryonic mouse primary neuron cultures to study possible upstream cellular mechanisms of cell death. Neuronal networks were grown on microelectrode array and electrical activity was recorded at different times post Zika virus infection. In addition to this method, we used confocal microscopy and Q-PCR techniques to observe morphological and molecular changes after infection.
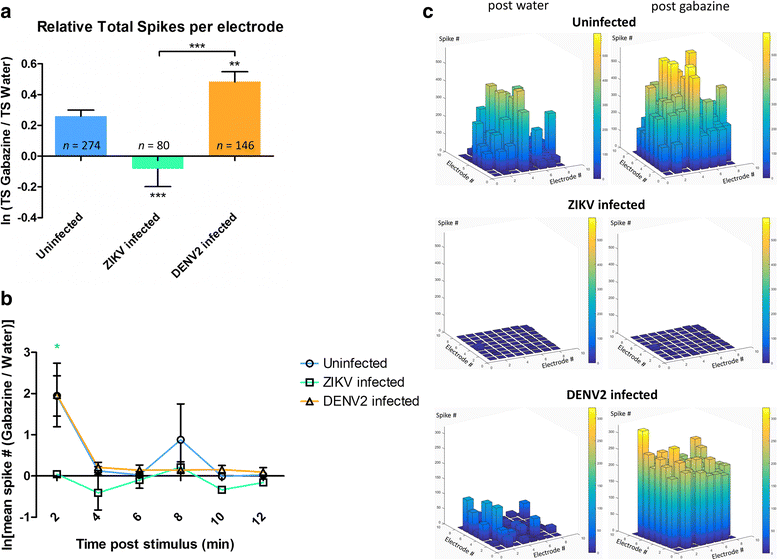
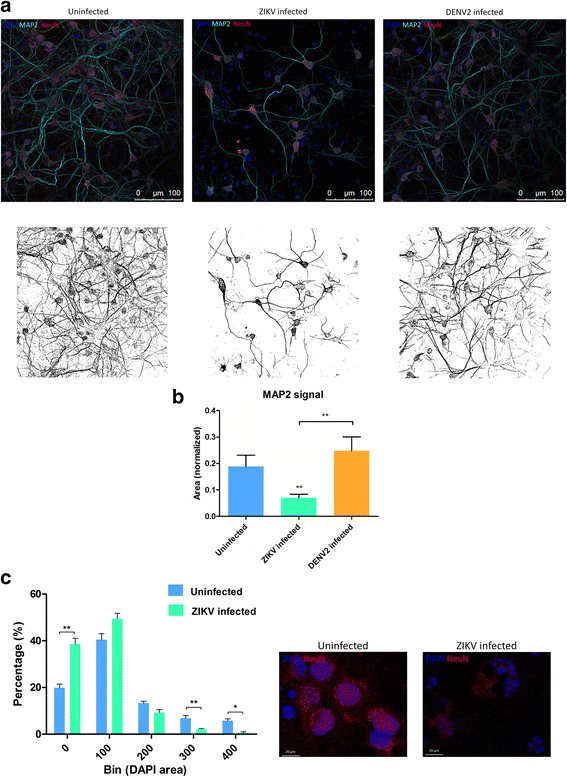
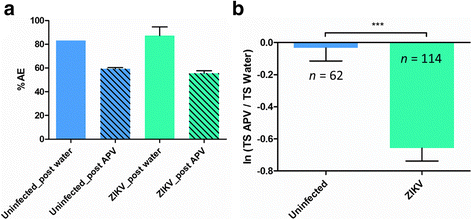
Results: Zika virus infection of mouse primary neurons triggers an early spiking excitation of neuron cultures, followed by dramatic loss of this activity. Using NMDA receptor antagonist, we show that this excitotoxicity mechanism, likely via glutamate, could also contribute to the observed nervous system defects in human embryos and could open new perspective regarding the causes of adult neuropathies.
Conclusions: This model of excitotoxicity, in the context of neurotropic virus infection, highlights the significance of neuronal activity recording with microelectrode array and possibility of more than one lethal mechanism after Zika virus infection in the nervous system.
Keywords: Excitotoxicity; Glutamate; Hyper excitation; Microelectrode array; Primary neuron; Spike; Zika.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau V, Broult J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19(14):20761. doi: 10.2807/1560-7917.ES2014.19.14.20761. - DOI - PubMed
-
- França GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, Nunes ML, Castro MC, Serruya S, Silveira MF. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388(10047):891–897. doi: 10.1016/S0140-6736(16)30902-3. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

