Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome
- PMID: 29703891
- PMCID: PMC5923383
- DOI: 10.1038/s41467-018-03770-3
Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome
Abstract
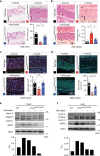
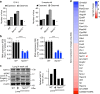
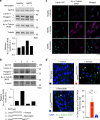
Hutchinson-Gilford Progeria Syndrome (HGPS) is a rare, but devastating genetic disease characterized by segmental premature aging, with cardiovascular disease being the main cause of death. Cells from HGPS patients accumulate progerin, a permanently farnesylated, toxic form of Lamin A, disrupting the nuclear shape and chromatin organization, leading to DNA-damage accumulation and senescence. Therapeutic approaches targeting farnesylation or aiming to reduce progerin levels have provided only partial health improvements. Recently, we identified Remodelin, a small-molecule agent that leads to amelioration of HGPS cellular defects through inhibition of the enzyme N-acetyltransferase 10 (NAT10). Here, we show the preclinical data demonstrating that targeting NAT10 in vivo, either via chemical inhibition or genetic depletion, significantly enhances the healthspan in a Lmna G609G HGPS mouse model. Collectively, the data provided here highlights NAT10 as a potential therapeutic target for HGPS.
Conflict of interest statement
D.L., S.P.J., and R.R. are named inventors on a patent describing compounds that include Remodelin. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

