Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1
- PMID: 29704429
- PMCID: PMC6766750
- DOI: 10.1002/jnr.24250
Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1
Abstract
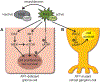
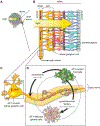
Neurofibromatosis type 1 (NF1) is a common cancer predisposition syndrome caused by mutations in the NF1 gene. The NF1-encoded protein (neurofibromin) is an inhibitor of the oncoprotein RAS and controls cell growth and survival. Individuals with NF1 are prone to developing low-grade tumors of the optic nerves, chiasm, tracts, and radiations, termed optic pathway gliomas (OPGs), which can cause vision loss. A paucity of surgical tumor specimens and of patient-derived xenografts for investigative studies has limited our understanding of human NF1-associated OPG (NF1-OPG). However, mice genetically engineered to harbor Nf1 gene mutations develop optic gliomas that share many features of their human counterparts. These genetically engineered mouse (GEM) strains have provided important insights into the cellular and molecular determinants that underlie mouse Nf1 optic glioma development, maintenance, and associated vision loss, with relevance by extension to human NF1-OPG disease. Herein, we review our current understanding of NF1-OPG pathobiology and describe the mechanisms responsible for tumor initiation, growth, and associated vision loss in Nf1 GEM models. We also discuss how Nf1 GEM and other preclinical models can be deployed to identify and evaluate molecularly targeted therapies for OPG, particularly as they pertain to future strategies aimed at preventing or improving tumor-associated vision loss in children with NF1.
Keywords: RAS; brain tumor; neurofibromatosis type 1; neurofibromin; optic nerve; optic pathway glioma; retinal ganglion cell; vision loss.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

References
-
- Alvord EC Jr., Lofton S. 1988. Gliomas of the optic nerve or chiasm. Outcome by patients’ age, tumor site, and treatment. J Neurosurg 68(1):85–98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

