Physiological activation and deactivation of soluble guanylate cyclase
- PMID: 29704567
- PMCID: PMC6919197
- DOI: 10.1016/j.niox.2018.04.011
Physiological activation and deactivation of soluble guanylate cyclase
Abstract
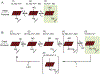
Soluble guanylate cyclase (sGC) is responsible for transducing the gaseous signaling molecule nitric oxide (NO) into the ubiquitous secondary signaling messenger cyclic guanosine monophosphate in eukaryotic organisms. sGC is exquisitely tuned to respond to low levels of NO, allowing cells to respond to non-toxic levels of NO. In this review, the structure of sGC is discussed in the context of sGC activation and deactivation. The sequence of events in the activation pathway are described into a comprehensive model of in vivo sGC activation as elucidated both from studies with purified enzyme and those done in cells. This model is then used to discuss the deactivation of sGC, as well as the molecular mechanisms of pathophysiological deactivation.
Keywords: Allosteric activation; Heme cofactor; Nitric oxide; Soluble guanylate cyclase.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of interest
The authors declare no competing financial interests.
Figures


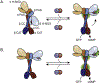
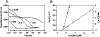
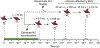

References
-
- Furchgott RF, Zawadzki JV, The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine, Nature 288 (1980) 373–376. - PubMed
-
- Palmer RM, Ferrige AG, Moncada S, Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor, Nature 327 (1987) 524–526. - PubMed
-
- Ignarro LJ, Byrns RE, Buga GM, Wood KS, Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical, Circ. Res 61 (1987) 866–879. - PubMed
-
- Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS, Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate, Biochemistry 27 (1988) 8706–8711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

