β-Hydroxybutyrate protects from alcohol-induced liver injury via a Hcar2-cAMP dependent pathway
- PMID: 29705237
- PMCID: PMC6098974
- DOI: 10.1016/j.jhep.2018.04.004
β-Hydroxybutyrate protects from alcohol-induced liver injury via a Hcar2-cAMP dependent pathway
Abstract
Background & aims: Sterile inflammation resulting in alcoholic hepatitis (AH) occurs unpredictably after many years of excess alcohol intake. The factors responsible for the development of AH are not known but mitochondrial damage with loss of mitochondrial function are common features. Hcar2 is a G-protein coupled receptor which is activated by β-hydroxybutyrate (BHB). We aimed to determine the relevance of the BHB-Hcar2 pathway in alcoholic liver disease.
Methods: We tested if loss of BHB production can result in increased liver inflammation. We further tested if BHB supplementation is protective in AH through interaction with Hcar2, and analyzed the immune and cellular basis for protection.
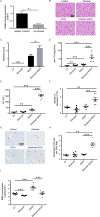
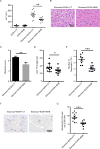
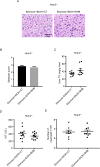
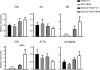
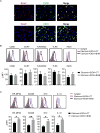
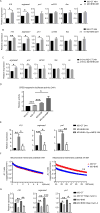
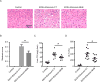
Results: Humans with AH have reduced hepatic BHB, and inhibition of BHB production in mice aggravated ethanol-induced AH, with higher plasma alanine aminotransferase levels, increased steatosis and greater neutrophil influx. Conversely supplementation of BHB had the opposite effects with reduced alanine aminotransferase levels, reduced steatosis and neutrophil influx. This therapeutic effect of BHB is dependent on the receptor Hcar2. BHB treatment increased liver Il10 transcripts, and promoted the M2 phenotype of intrahepatic macrophages. BHB also increased the transcriptional level of M2 related genes in vitro bone marrow derived macrophages. This skewing towards M2 related genes is dependent on lower mitochondrial membrane potential (Δψ) induced by BHB.
Conclusions: Collectively, our data shows that BHB production during excess alcohol consumption has an anti-inflammatory and hepatoprotective role through an Hcar2 dependent pathway. This introduces the concept of metabolite-based therapy for AH.
Lay summary: Alcoholic hepatitis is a life-threatening condition with no approved therapy that occurs unexpectedly in people who consume excess alcohol. The liver makes many metabolites, and we demonstrate that loss of one such metabolite β-hydroxybutyrate occurs in patients with alcoholic hepatitis. This loss can increase alcohol-induced liver injury, and β-hydroxybutyrate can protect from alcohol-induced liver injury via a receptor on liver macrophages. This opens the possibility of metabolite-based therapy for alcoholic hepatitis.
Keywords: Alcohol-induced liver injury; Hcar2; Therapy; β-Hydroxybutyrate.
Copyright © 2018 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
References
-
- Crabb DW, Liangpunsakul S. Alcohol and lipid metabolism. J Gastroenterol Hepatol. 2006;21(Suppl 3):S56–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

