Transfer of gene-corrected T cells corrects humoral and cytotoxic defects in patients with X-linked lymphoproliferative disease
- PMID: 29705247
- PMCID: PMC6034012
- DOI: 10.1016/j.jaci.2018.02.053
Transfer of gene-corrected T cells corrects humoral and cytotoxic defects in patients with X-linked lymphoproliferative disease
Abstract
Background: X-linked lymphoproliferative disease 1 arises from mutations in the SH2D1A gene encoding SLAM-associated protein (SAP), an adaptor protein expressed in T, natural killer (NK), and NKT cells. Defects lead to abnormalities of T-cell and NK cell cytotoxicity and T cell-dependent humoral function. Clinical manifestations include hemophagocytic lymphohistiocytosis, lymphoma, and dysgammaglobulinemia. Curative treatment is limited to hematopoietic stem cell transplantation, with outcomes reliant on a good donor match.
Objectives: Because most symptoms arise from defective T-cell function, we investigated whether transfer of SAP gene-corrected T cells could reconstitute known effector cell defects.
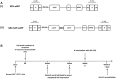
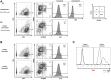
Methods: CD3+ lymphocytes from Sap-deficient mice were transduced with a gammaretroviral vector encoding human SAP cDNA before transfer into sublethally irradiated Sap-deficient recipients. After immunization with the T-dependent antigen 4-hydroxy-3-nitrophenylacetly chicken gammaglobulin (NP-CGG), recovery of humoral function was evaluated through germinal center formation and antigen-specific responses. To efficiently transduce CD3+ cells from patients, we generated an equivalent lentiviral SAP vector. Functional recovery was demonstrated by using in vitro cytotoxicity and T follicular helper cell function assays alongside tumor clearance in an in vivo lymphoblastoid cell line lymphoma xenograft model.
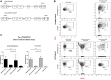
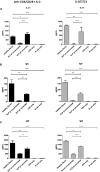
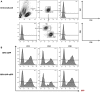
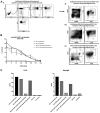
Results: In Sap-deficient mice 20% to 40% engraftment of gene-modified T cells led to significant recovery of germinal center formation and NP-specific antibody responses. Gene-corrected T cells from patients demonstrated improved cytotoxicity and T follicular helper cell function in vitro. Adoptive transfer of gene-corrected cytotoxic T lymphocytes from patients reduced tumor burden to a level comparable with that seen in healthy donor cytotoxic T lymphocytes in an in vivo lymphoma model.
Conclusions: These data demonstrate that autologous T-cell gene therapy corrects SAP-dependent defects and might offer an alternative therapeutic option for patients with X-linked lymphoproliferative disease 1.
Keywords: T-cell cytotoxicity; T-cell gene therapy; X-linked lymphoproliferative disease; follicular helper T cells.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Parolini S., Bottino C., Falco M., Augugliaro R., Giliani S., Franceschini R. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192:337–346. - PMC - PubMed
-
- Tangye S.G., Phillips J.H., Lanier L.L., Nichols K.E. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J Immunol. 2000;165:2932–2936. - PubMed
-
- Dupre L., Andolfi G., Tangye S.G., Clementi R., Locatelli F., Arico M. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 2005;105:4383–4389. - PubMed
-
- Nakajima H., Cella M., Bouchon A., Grierson H.L., Lewis J., Duckett C.S. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur J Immunol. 2000;30:3309–3318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

