Direct mechanical thrombectomy in tPA-ineligible and -eligible patients versus the bridging approach: a meta-analysis
- PMID: 29705773
- PMCID: PMC6327861
- DOI: 10.1136/neurintsurg-2018-013834
Direct mechanical thrombectomy in tPA-ineligible and -eligible patients versus the bridging approach: a meta-analysis
Abstract
Background: Whether pretreatment with intravenous thrombolysis prior to mechanical thrombectomy (IVT+MTE) adds additional benefit over direct mechanical thrombectomy (dMTE) in patients with large vessel occlusions (LVO) is a matter of debate.
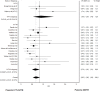
Methods: This study-level meta-analysis was presented in accord with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Pooled effect sizes were calculated using the inverse variance heterogeneity model and displayed as summary Odds Ratio (sOR) and corresponding 95% confidence interval (95% CI). Sensitivity analysis was performed by distinguishing between studies including dMTE patients eligible for IVT (IVT-E) or ineligible for IVT (IVT-IN). Primary outcome measures were functional independence (modified Rankin Scale≤2) and mortality at day 90, successful reperfusion, and symptomatic intracerebral hemorrhage.
Results: Twenty studies, incorporating 5279 patients, were included. There was no evidence that rates of successful reperfusion differed in dMTE and IVT+MTE patients (sOR 0.93, 95% CI 0.68 to 1.28). In studies including IVT-IN dMTE patients, patients undergoing dMTE tended to have lower rates of functional independence and had higher odds for a fatal outcome as compared with IVT+MTE patients (sOR 0.78, 95% CI 0.61 to 1.01 and sOR 1.45, 95% CI 1.22 to 1.73). However, no such treatment group effect was found when analyses were confined to cohorts with a lower risk of selection bias (including IVT-E dMTE patients).
Conclusion: The quality of evidence regarding the relative merits of IVT+MTE versus dMTE is low. When considering studies with lower selection bias, the data suggest that dMTE may offer comparable safety and efficacy as compared with IVT+MTE. The conduct of randomized-controlled clinical trials seems justified.
Keywords: stroke; thrombectomy; thrombolysis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2019. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: Related: UF and JG are global PIs for the SWIFT DIRECT trial and are consultants for Medtronic. VMP is a PI for the SWIFT DIRECT trial and is a consultant for Medtronic. Unrelated: MA received honoraria for lectures and advisory boards from Bayer, Boehringer Ingelheim, Bristol Meyer Squibbs, Pfizer, and Covidien. MG is a consultant for Medtronic, Stryker, Microvention/ and Ablynx and received grants from Medtronic and Stryker provided to the University of Calgary. He has a licensing agreement with GE for systems of stroke diagnosis. MDH received a grant from Alberta Innovates for stroke program in Alberta. VMP is a consultant for Stryker (SC for DAWN trial), Penumbra (SC for PROMISE study), BALT (proctorship of products unrelated to ischemic stroke), Phenox, Rapid Medical, Neurovasc and receives research a grant from Philips. JLS is a consultant about trial design and conduct for Covidien and Stryker, and employee of the University of California, which holds a patent on retriever devices for stroke. JG is a global PI of STAR, CEC member of the PROMISE study (Penumbra), Consultancy; and receives SNSF grants for magnetic resonance imaging in stroke. UF receives research grants from Swiss National Science Foundation (SNSF). All other authors have nothing to disclose.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
