Neuropsychological Profiles and Trajectories in Preclinical Alzheimer's Disease
- PMID: 29706146
- PMCID: PMC6108905
- DOI: 10.1017/S135561771800022X
Neuropsychological Profiles and Trajectories in Preclinical Alzheimer's Disease
Abstract
Objectives: The present study investigated the independent and synergistic effects of amyloid beta (Aβ1-42) and phosphorylated tau (Ptau) pathologies on neuropsychological profiles and trajectories in cognitively normal older adults.
Methods: Alzheimer's Disease Neuroimaging Initiative participants identified as cognitively normal at baseline underwent longitudinal assessment (N=518; 0, 12, 24, 36 months), baseline lumbar puncture and follow-up cognitive exams. Cerebral spinal fluid (CSF) biomarker profiles (Aβ-Ptau-, Aβ+Ptau-, Aβ-Ptau+, Aβ+Ptau+) were compared on baseline profiles and trajectories for memory (Rey Auditory Verbal Learning Test), attention/executive function (Trail Making Test, A and B), language (Animal Fluency, Vegetable Fluency, Boston Naming Test) and processing speed (Digit Symbol) using multilevel models.
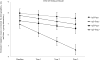
Results: The Aβ+Ptau+ group exhibited significantly worse baseline performance on tests of memory and executive function relative to the Aβ-Ptau+ and Aβ-Ptau- groups. The Aβ+Ptau- group fell between the Aβ+Ptau+ participants and the Aβ-Ptau- and Aβ-Ptau+ groups on all three cognitive domains and exhibited worse baseline executive function. The Aβ-Ptau+ group performed worse than Aβ-Ptau- participants on processing speed. Over 36-month follow-up, the Aβ+Ptau+ group exhibited the greatest declines in memory and semantic fluency compared to all other groups.
Conclusions: Cognitively normal older adults with both Aβ and Ptau pathology exhibited the weakest profile, marked by the worst memory decline compared to the other groups. Other subtle changes in this group included declines in executive function and semantic fluency. Those with Ptau pathology alone showed slowed processing speed, and those with Aβ pathology alone showed worse attention and executive function compared to biomarker negative participants. (JINS, 2018, 24, 693-702).
Keywords: Alzheimer’s disease; Amyloid beta; Biomarkers; Cognition; Memory; Tau.
Conflict of interest statement
Figures




References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Petersen RC. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s Disease. Alzheimer’s & Dementia. 2011;7(3):270–279. - PMC - PubMed
-
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. Journal of Comparative Neurology. 2005;493(1):99–110. - PubMed
-
- Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, Eichenlaub U. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1-42) in human cerebrospinal fluid. Alzheimer’s & Dementia. 2016;12(5):517–526. - PubMed
-
- Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. The Lancet Neurology. 2003;2(10):605–613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

