Extrinsic Phagocyte-Dependent STING Signaling Dictates the Immunogenicity of Dying Cells
- PMID: 29706455
- PMCID: PMC6177226
- DOI: 10.1016/j.ccell.2018.03.027
Extrinsic Phagocyte-Dependent STING Signaling Dictates the Immunogenicity of Dying Cells
Abstract
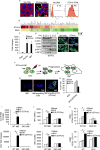
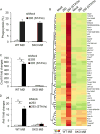
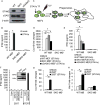
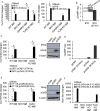
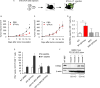
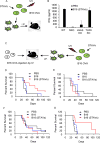
The ability of dying cells to activate antigen-presenting cells (APCs) is carefully controlled to avoid unwarranted inflammatory responses. Here, we show that engulfed cells containing cytosolic double-stranded DNA species (viral or synthetic) or cyclic di-nucleotides (CDNs) are able to stimulate APCs via extrinsic STING (stimulator of interferon genes) signaling, to promote antigen cross-presentation. In the absence of STING agonists, dying cells were ineffectual in the stimulation of APCs in trans. Cytosolic STING activators, including CDNs, constitute cellular danger-associated molecular patterns (DAMPs) only generated by viral infection or following DNA damage events that rendered tumor cells highly immunogenic. Our data shed insight into the molecular mechanisms that drive appropriate anti-tumor adaptive immune responses, while averting harmful autoinflammatory disease, and provide a therapeutic strategy for cancer treatment.
Keywords: STING; STING-dependent adjuvants (STAVs); anti-tumor T cells; antigen-presenting cells (APCs); cyclic di-nucleotides (CDNs); innate immunity; interferon.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
The authors have a patent related to this work. Application number: 15/120,694/Undergoing Examination.
Figures

References
-
- Ahn J, Barber GN. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr Opin Immunol. 2014;31:121–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

