Dendritic Integration of Sensory Evidence in Perceptual Decision-Making
- PMID: 29706545
- PMCID: PMC5947940
- DOI: 10.1016/j.cell.2018.03.075
Dendritic Integration of Sensory Evidence in Perceptual Decision-Making
Abstract
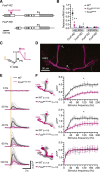
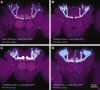
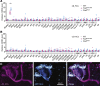
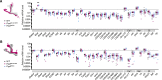
Perceptual decisions require the accumulation of sensory information to a response criterion. Most accounts of how the brain performs this process of temporal integration have focused on evolving patterns of spiking activity. We report that subthreshold changes in membrane voltage can represent accumulating evidence before a choice. αβ core Kenyon cells (αβc KCs) in the mushroom bodies of fruit flies integrate odor-evoked synaptic inputs to action potential threshold at timescales matching the speed of olfactory discrimination. The forkhead box P transcription factor (FoxP) sets neuronal integration and behavioral decision times by controlling the abundance of the voltage-gated potassium channel Shal (KV4) in αβc KC dendrites. αβc KCs thus tailor, through a particular constellation of biophysical properties, the generic process of synaptic integration to the demands of sequential sampling.
Keywords: Drosophila melanogaster; decision-making; drift-diffusion model; forkhead box P transcription factors; membrane biophysics; neural integrator; olfaction; potassium channel; synaptic integration.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures











Comment in
-
Decision Making: How Fruit Flies Integrate Olfactory Evidence.Curr Biol. 2018 Jul 9;28(13):R757-R759. doi: 10.1016/j.cub.2018.05.065. Curr Biol. 2018. PMID: 29990462
References
-
- Aso Y., Grübel K., Busch S., Friedrich A.B., Siwanowicz I., Tanimoto H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 2009;23:156–172. - PubMed
-
- Awasaki T., Saito M., Sone M., Suzuki E., Sakai R., Ito K., Hama C. The Drosophila Trio plays an essential role in patterning of axons by regulating their directional extension. Neuron. 2000;26:119–131. - PubMed
-
- Bogacz R., Brown E., Moehlis J., Holmes P., Cohen J.D. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 2006;113:700–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

